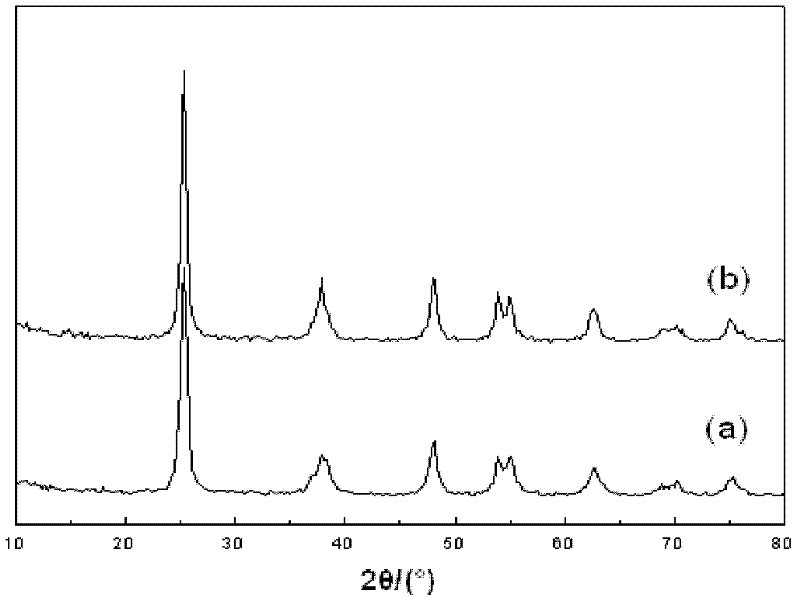
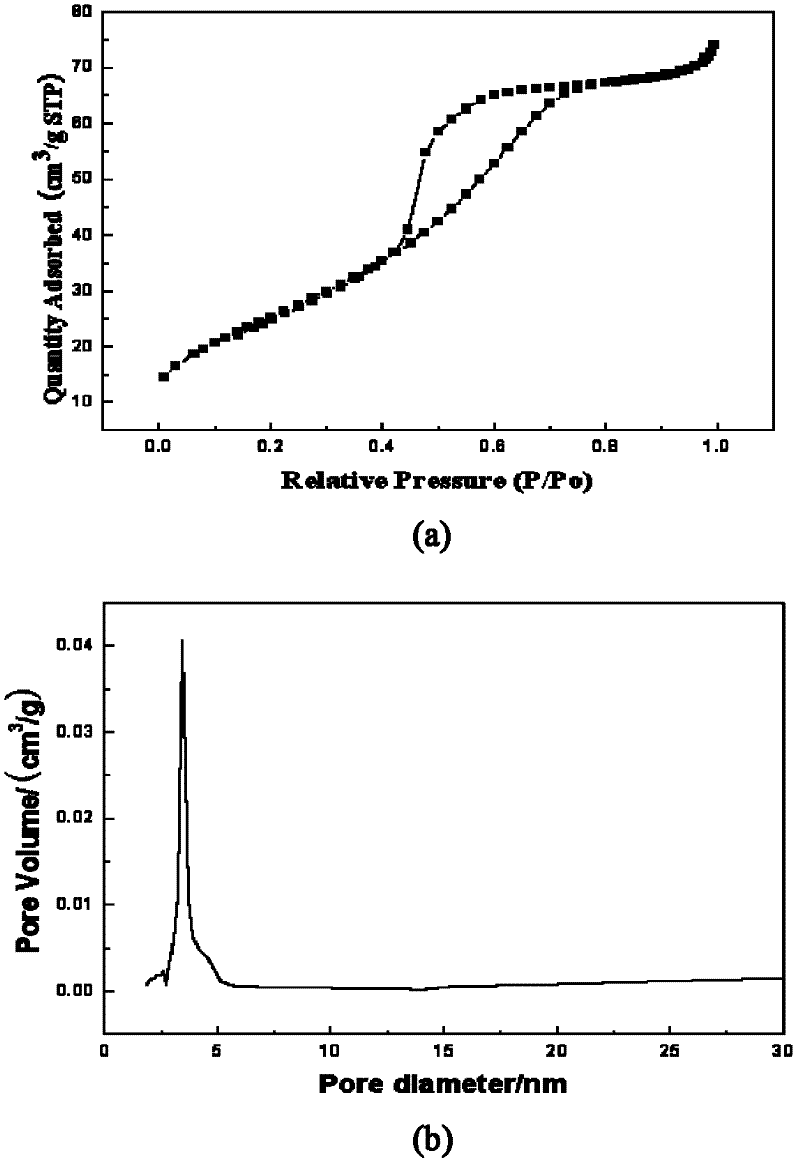
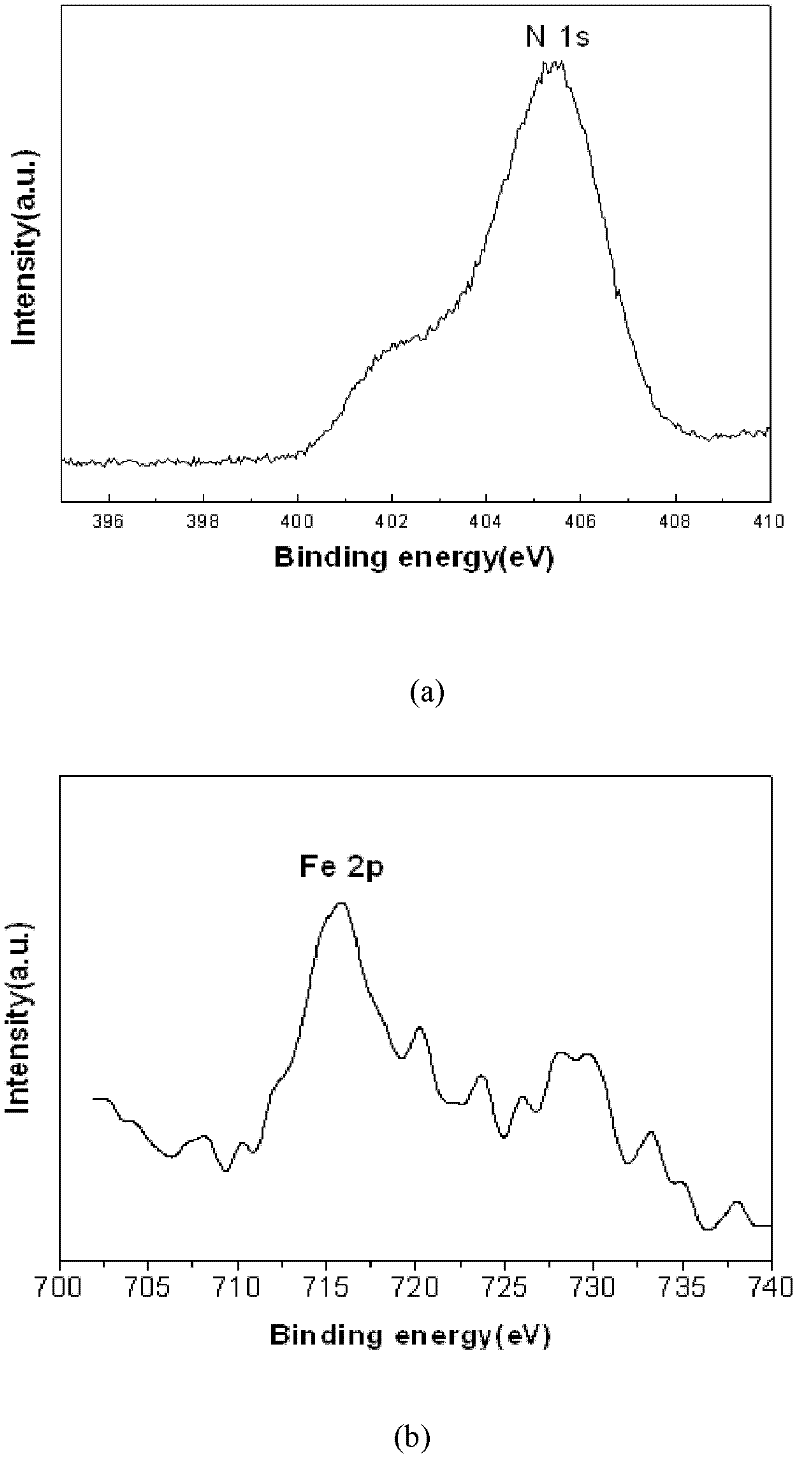
Method for fast sol-gel preparation of iron-nitrogen co-doped mesoporous nano-titanium dioxide
A nano-titanium dioxide, sol-gel technology, applied in titanium dioxide, titanium oxide/hydroxide, nanotechnology and other directions, can solve the problem of reducing the recombination rate of photogenerated electron-hole pairs, and achieve iron-nitrogen co-doped mesoporous Nano titanium dioxide UV-visible absorption edge red shift is obvious, the electron-hole recombination rate is reduced, and the operation steps are simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) At room temperature, add 280mL of absolute ethanol, 10mL of deionized water, 1.5g of urea, and 0.12g of ferric nitrate nonahydrate into a beaker, then use 5wt% dilute nitric acid to adjust the pH to 3-4, stir, and record as A;
[0028] (2) Take a small beaker, add 20mL absolute ethanol and 10mL butyl titanate respectively, mix well, record as B;
[0029] (3) Take 0.1g polyacrylamide (molecular weight: 3 million) and add it into 100g deionized water to prepare a solution with a mass concentration of about 0.1wt%, denoted as C;
[0030] (4) Slowly drop A into B, and keep stirring. After the addition, continue to stir for 10-15 minutes, and then add 20mL of C solution dropwise. Continue to stir for 1 hour after the addition to obtain a wet gel;
[0031] (5) Put the wet gel in an oven and dry it at 80°C to remove the solvent and moisture to obtain the wet gel;
[0032] (6) Grind the xerogel in a mortar for 10 minutes, take it out and put it into a crucible, calcinate...
Embodiment 2
[0034] (1) At room temperature, add 200 mL of absolute ethanol, 10 mL of deionized water, 1.0 g of urea, and 0.05 g of ferric nitrate nonahydrate into a beaker, and then use 5 wt % dilute nitric acid to adjust the pH to 3 to 4, which is designated as A, stir;
[0035] (2) Take a small beaker, add 20mL absolute ethanol and 10mL butyl titanate respectively, mix well, record as B;
[0036] (3) Get 15g polyethylene glycol (molecular weight is 20,000) and add 100g deionized water, the preparation mass concentration is about the polyethylene glycol solution of 15wt%, denoted as C;
[0037] (4) Slowly drop A into B, and keep stirring, continue to stir for 10 minutes after the addition, and then slowly add 20mL of C solution dropwise. Continue to stir for 2h after adding until gelling;
[0038] (5) Put the wet gel in an oven and dry it at 90°C to remove the solvent and moisture to obtain a dry gel;
[0039] (6) Grind the xerogel in a mortar for 15 minutes, take it out and put it in...
Embodiment 3
[0041] This embodiment is specifically carried out according to the following steps:
[0042]1) At room temperature, first use dilute nitric acid or dilute hydrochloric acid to adjust the mixed solution of absolute ethanol, deionized water, nitrogen source, and iron source to a pH value of 3 to 4, wherein the volume ratio of absolute ethanol to water is 20:1 , then drop the absolute ethanol solution of butyl titanate at a volume ratio of 1:1, and then add an aqueous solution of polyacrylamide with a mass fraction of 0.1%, and stir for 0.1 h to obtain a gel; wherein the nitrogen source is urea, The molar ratio of titanium source to urea is 0.90:1; the iron source is ferric nitrate nonahydrate, and the molar ratio of titanium source to iron source is 1:0.001;
[0043] 2) Dry the gel in an oven at 80°C for 4 hours to remove the solvent and water to obtain a xerogel; grind the xerogel in a mortar, put it in a crucible, and calcinate and remove the template agent at a low temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com