Method for preparing ethyl eicosapentaenate (EPA) and ethyl docosahexaenoate (DHA)
An ethyl ester and preparation type technology is applied in the field of preparation of EPA ethyl ester and DHA ethyl ester, and can solve the problems of inability to achieve simultaneous separation, high toxicity of acetonitrile, pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
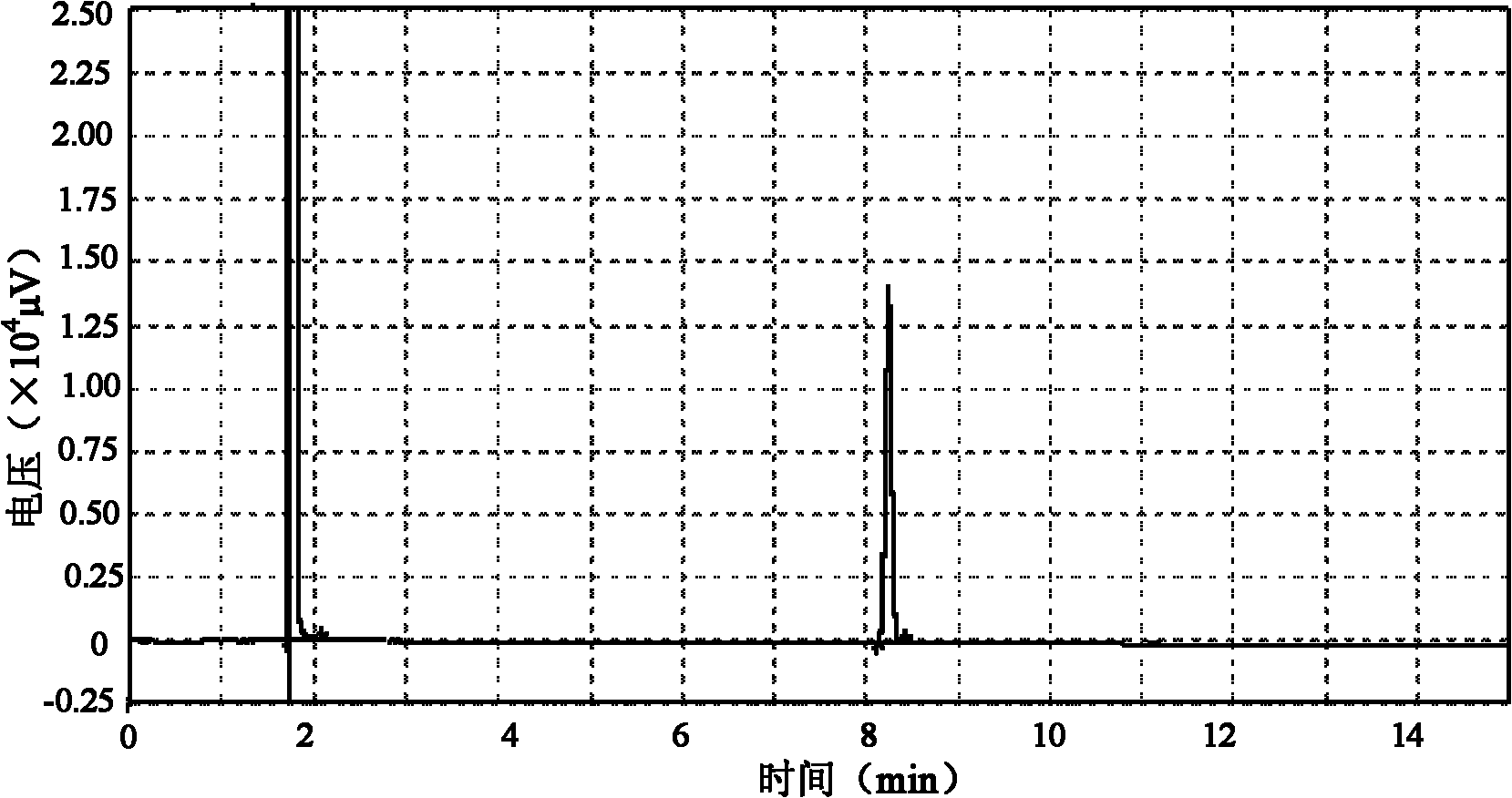
Embodiment 1
[0018] Preparation of raw materials: the crude fish oil with a total content of EPA ethyl ester and DHA ethyl ester of 80% is prepared into a pre-separated preparation solution containing 200 mg of the crude product per ml, and set aside.
[0019] Instrument: Preparative high performance liquid chromatography-mass spectrometry instrument system.
[0020] Chromatographic conditions: the preparation chromatographic column is a C18 column (250mm×20mm); the mobile phase system is 90% methanol aqueous solution, and the flow rate of the mobile phase is 20mL / min.
[0021] Injection concentration: 200mg / mL.
[0022] Injection volume: 1 mL.
[0023] Mass spectrometry detection conditions: atmospheric pressure chemical ionization source (APCI), positive ion mode, corona current 3.0μA, primary cone voltage 30V, secondary cone voltage 3.0V, source temperature 120°C, desolvation temperature 350°C, desolvation nitrogen flow rate 300L / h, cone nitrogen flow rate 50L / h, high and low mass res...
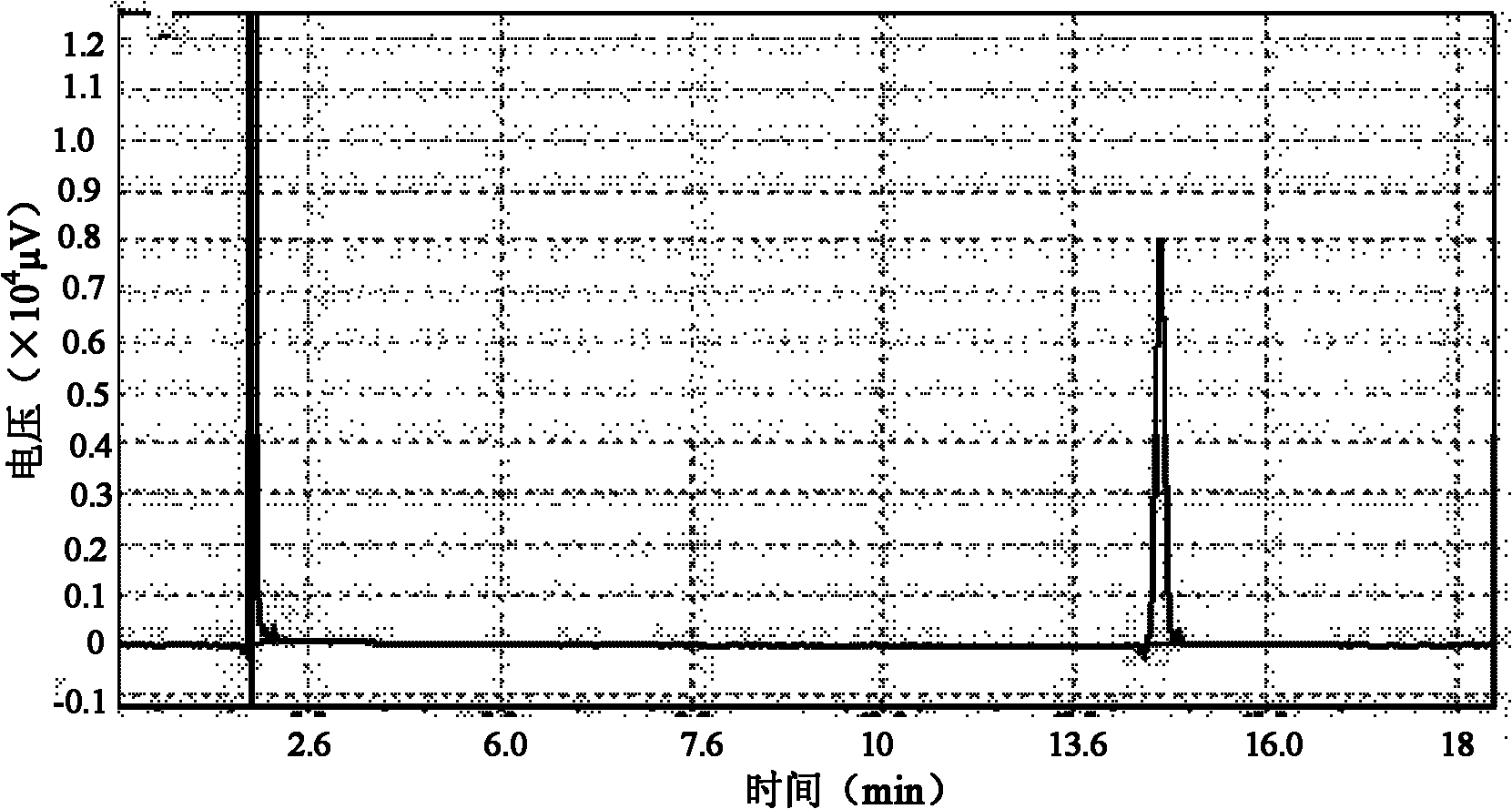
Embodiment 2
[0026] Raw materials for preparation: the crude fish oil with a total content of EPA ethyl ester and DHA ethyl ester of 70% is prepared into a pre-separated preparation solution containing 150 mg of the crude product per milliliter, and set aside.
[0027] Instrument: Preparative high performance liquid chromatography-mass spectrometry instrument system.
[0028] Chromatographic conditions: the preparation chromatographic column is a C8 column (250mm×20mm); the mobile phase system is 85% methanol aqueous solution, and the flow rate of the mobile phase is 20mL / min.
[0029] Injection concentration: 150mg / mL.
[0030] Injection volume: 1.5mL.
[0031] Mass spectrometry detection conditions: atmospheric pressure chemical ionization source (APCI), positive ion mode, corona current 3.0μA, primary cone voltage 30V, secondary cone voltage 3.0V, source temperature 120°C, desolvation temperature 350°C, desolvation nitrogen flow rate 300L / h, cone nitrogen flow rate 50L / h, high and low...
Embodiment 3
[0034] Preparation of raw materials: the crude fish oil with a total content of EPA ethyl ester and DHA ethyl ester of 60% is prepared into a pre-separated preparation solution containing 200 mg of the crude product per ml, and set aside.
[0035] Instrument: Preparative high performance liquid chromatography-mass spectrometry instrument system.
[0036]Chromatographic conditions: the preparation chromatographic column is a C18 column (250mm×30mm); the mobile phase system is 85% ethanol aqueous solution, and the flow rate of the mobile phase is 45mL / min.
[0037] Injection concentration: 200mg / mL.
[0038] Injection volume: 2.5mL.
[0039] Mass spectrometry detection conditions: atmospheric pressure chemical ionization source (APCI), positive ion mode, corona current 3.0μA, primary cone voltage 30V, secondary cone voltage 3.0V, source temperature 120°C, desolvation temperature 350°C, desolvation nitrogen flow rate 300L / h, cone nitrogen flow rate 50L / h, high and low mass reso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com