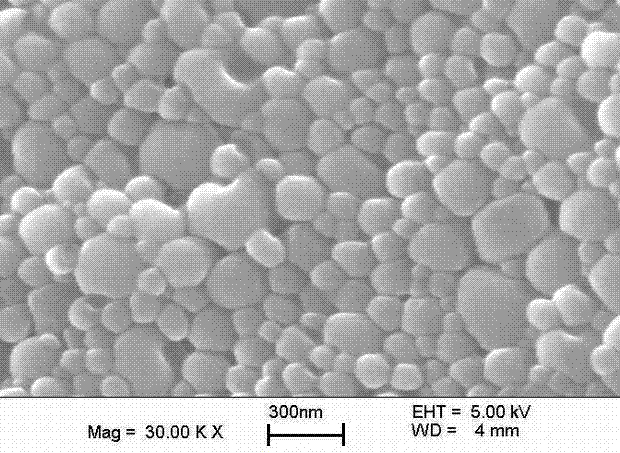
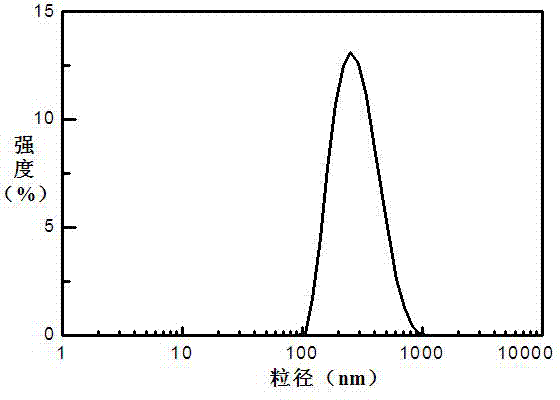
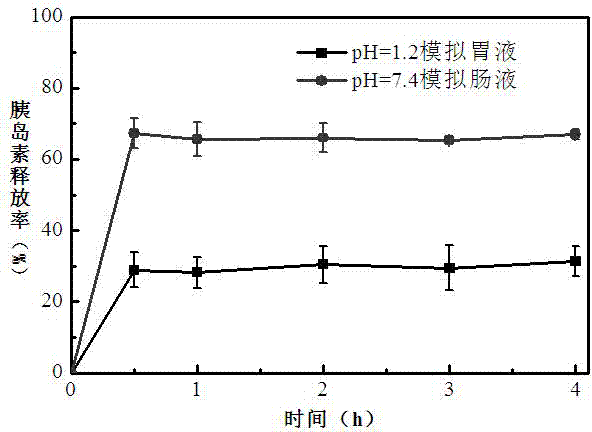
Oral PEGylated insulin pH-sensitive naonparticle and preparation method thereof
A technology of PEGylation and polyethylene glycol butyraldehyde, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem that the encapsulation rate is only 33~49.2%, Poor drug absorption ability, no pH sensitivity and other problems, to achieve the effect of good pH sensitive response release performance, reduced release, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Phacoemulsification to prepare W / O colostrum of PEGylated insulin, pH-sensitive polymer and carrier material: Weigh 40 mg of starch and dissolve it in 40 mL of deionized water to obtain a starch solution with a mass fraction of 0.1%. PEGylated insulin was dissolved in 0.5 mL of the above starch solution to form an internal aqueous phase; 1 mL of acetone and 4 mL of methylene chloride were mixed, and 50 mg of hydroxypropylmethylcellulose phthalate (abbreviation: HP55) was added to it Dissolve with 50mg polylactide (abbreviation: PLGA) to form an oil phase; mix 0.5mL internal water phase with 5mL oil phase, and ultrasonically emulsify at 40W for 0.5min to obtain W / O colostrum;
[0036] (2) Ultrasonic dispersion of W / O colostrum in the stabilizer solution to form W / O / W double emulsion: Weigh 100mg of polyvinyl alcohol (abbreviation: PVA), dissolve it in 10mL of deionized water, and form a PVA mass fraction of 1% The external water phase; mix 10mL of the external water ...
Embodiment 2
[0041] (1) Phacoemulsification to prepare W / O colostrum of PEGylated insulin, pH-sensitive polymer and carrier material: Weigh 200 mg of glycerol and add it into 40 mL of deionized water to obtain a glycerol solution with a mass fraction of 0.5%. PEGylated insulin was dissolved in 0.5mL of the above glycerin solution to form an internal water phase; 1mL of acetone and 4mL of dichloromethane were mixed, and 100mg of HP55 and 100mg of polylactic acid (PLA) were added to dissolve to form an oil phase; 0.5 Mix mL internal water phase and 5 mL oil phase, and ultrasonically emulsify at 40W for 4min to obtain W / O colostrum;
[0042] (2) Ultrasonic dispersion of W / O colostrum in stabilizer solution to form W / O / W double emulsion: Weigh 600mg of PVA and dissolve it in 20mL of deionized water to form an external aqueous phase with a mass fraction of PVA of 3%; Mix the external water phase with 5.5mL of W / O colostrum obtained in step (1), and ultrasonically emulsify at 60W for 2min to obt...
Embodiment 3
[0047] (1) Prepare W / O colostrum of PEGylated insulin, pH-sensitive polymer and carrier material by ultrasonic emulsification: Weigh 0.1 g of starch and dissolve it in 40 mL of deionized water to obtain a starch solution with a mass fraction of 0.25%. 10 mg of pegylated insulin was dissolved in 0.5 mL of the above starch solution to form an internal water phase; 1 mL of acetone and 4 mL of methylene chloride were mixed, and 100 mg of HP55 and 50 mg of PLGA were added to dissolve to form an oil phase; 0.5 mL of the internal water phase Mix with 5mL oil phase, 50W ultrasonic emulsification for 4min, get W / O colostrum;
[0048] (2) Ultrasonic dispersion of W / O colostrum in stabilizer solution to form W / O / W double emulsion: Weigh 0.8g of PVA and dissolve it in 40mL of deionized water to form an external aqueous phase with a mass fraction of PVA of 2%; Mix 40mL of the external water phase with 5.5mL of the W / O colostrum obtained in step (1), and ultrasonically emulsify at 50W for 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com