Preparation method of chitosan-loaded capsaicin microspheres and application of microspheres in weight loss, fat-lowering and blood sugar-lowering
A technology of chitosan microspheres and capsaicin, which is applied in the application, food preparation, pharmaceutical formulation and other directions, can solve the problems of strong irritant effect of CAP, no technical report, etc., achieve excellent sustained release performance, improve compliance, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation and detection experiment of CTS-CAP-MP
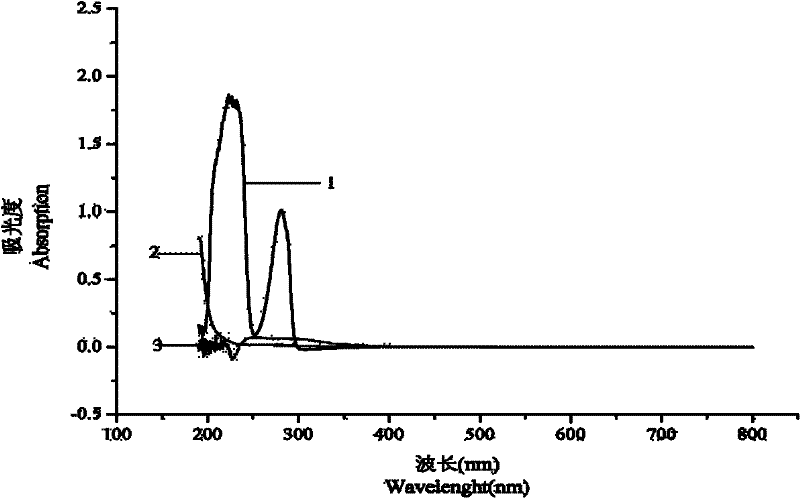
[0060] 1. Determination of the maximum absorption wavelength of CAP
[0061] In the wavelength range of 190-800nm, CAP, CTS and TPP are scanned at full wavelength, and the optimal absorption wavelength of CAP is selected to reduce the interference of excipients. attached figure 1 It is the ultraviolet full-wavelength scanning diagram of CAP, CTS and TPP, wherein curves 1, 2 and 3 represent the ultraviolet full-wavelength scanning diagram of CAP, TPP and CTS respectively. From attached figure 1 It is found that CAP has strong ultraviolet absorption at 239nm and 281nm and CTS and TPP have very little ultraviolet absorption at this wavelength. After analysis and summary, in order to avoid the influence of terminal absorption, the wavelength of ultraviolet absorption measurement of CAP is selected as 281nm.
[0062] 2. Preparation of CTS-CAP-MP by ionic cross-linking
[0063] Weigh 0.10 g of CTS and fully di...
Embodiment 2
[0089] Example 2 CTS-CAP-MP prescription optimization experiment
[0090] 1. Single factor experiment of CTS concentration
[0091] Under the conditions that the mass of fixed CAP added was 1.5 mg, the concentration of TPP was 1 mg / ml, the pH value was 4.5, and the mass ratio of CTS / TPP was 5:1, the concentrations of CTS were selected to be 0.5 mg / ml, 1.0 mg / ml, 1.5 mg / ml, 2.0mg / ml, 2.5mg / ml and 3.0mg / ml, the impact of CTS concentration on drug loading was investigated by measuring the encapsulation efficiency as the evaluation index. The experimental results of the effect of CTS concentration on the encapsulation efficiency are shown in the appendix Figure 6 shown. attached by Figure 6 It can be seen that as the concentration of CTS increases, the encapsulation efficiency of CAP in the solution will also increase accordingly. However, according to the analysis of the present invention, if the CTS concentration is too high and the viscosity is high, the wall sticking phe...
Embodiment 3
[0116] Example 3 Preparation process optimization experiment of CTS-CAP-MP microspheres
[0117] 1. Selection of inlet air temperature
[0118] In hot air volume 36m 3 / h, the injection rate is 400ml / h, the compressed air pressure is 10l / min, and the inlet temperature of the instrument is set at 140°C, 160°C, and 180°C respectively for single-factor comparative experiments. The experimental results are evaluated by the yield and particle size , investigate the experimental effect of different inlet temperatures, and select the appropriate inlet temperature range. Comprehensive investigation and analysis of the influence of air inlet temperature on the particle size and yield of CTS-CAP-MP, the experimental results are shown in Table 9. The data showed that inlet air temperature had little effect on particle size differences, but had an effect on yield. The yield difference between 160°C (59.6%) and 180°C (59.8%) is not much different, but considering factors such as higher ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com