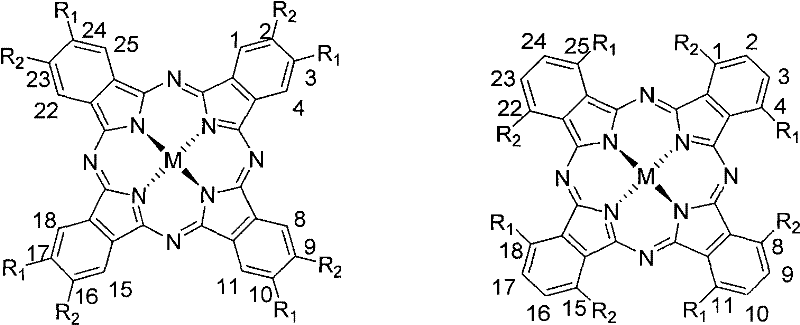
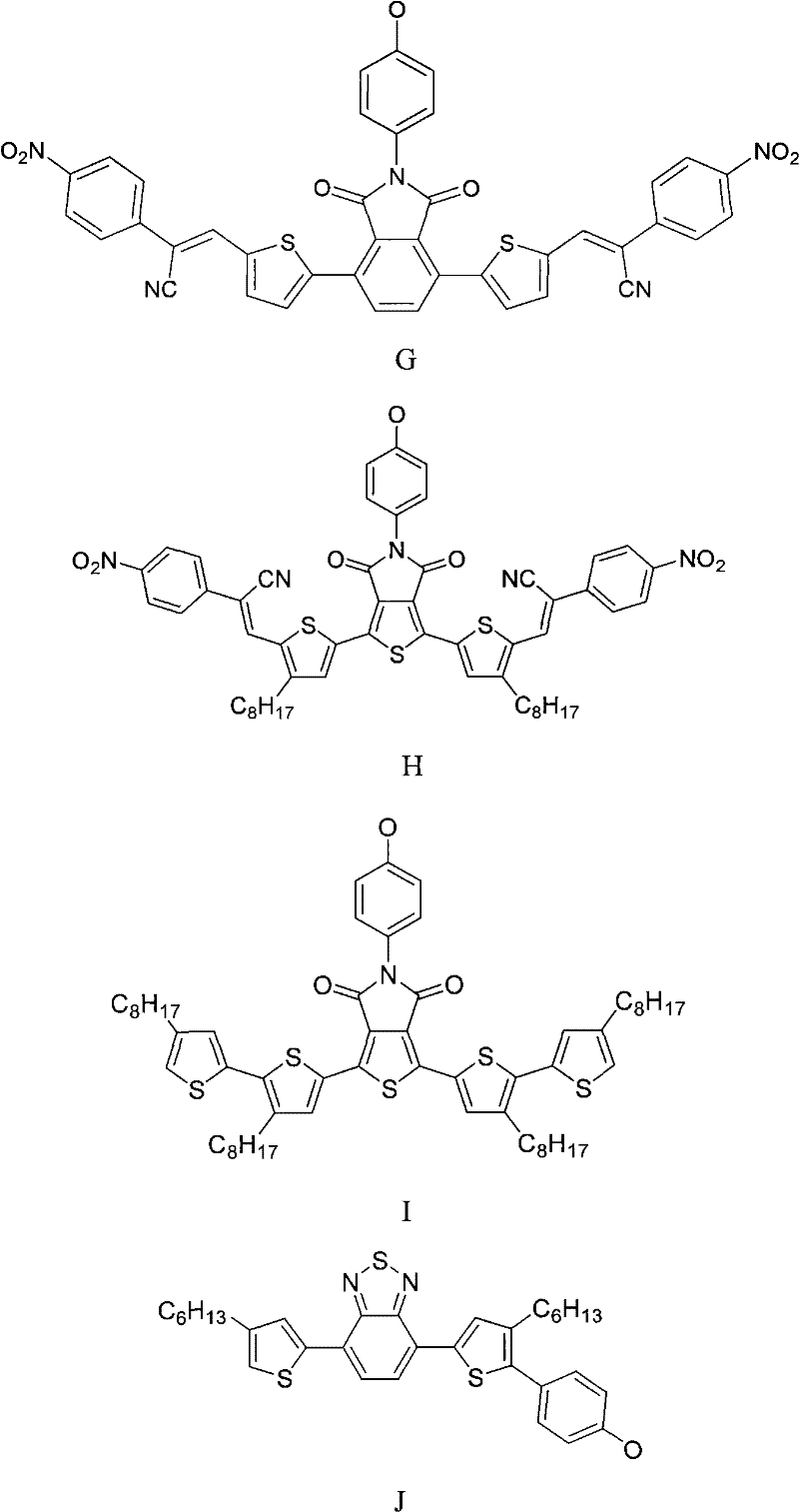
Organic-functionalized non-aggregated phthalocyanine and preparation method thereof
A functional, non-aggregated technology, applied in the field of material science, can solve the problems of high raw material cost, high cost, unfavorable commercial application, etc., and achieve the effect of broad absorption spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
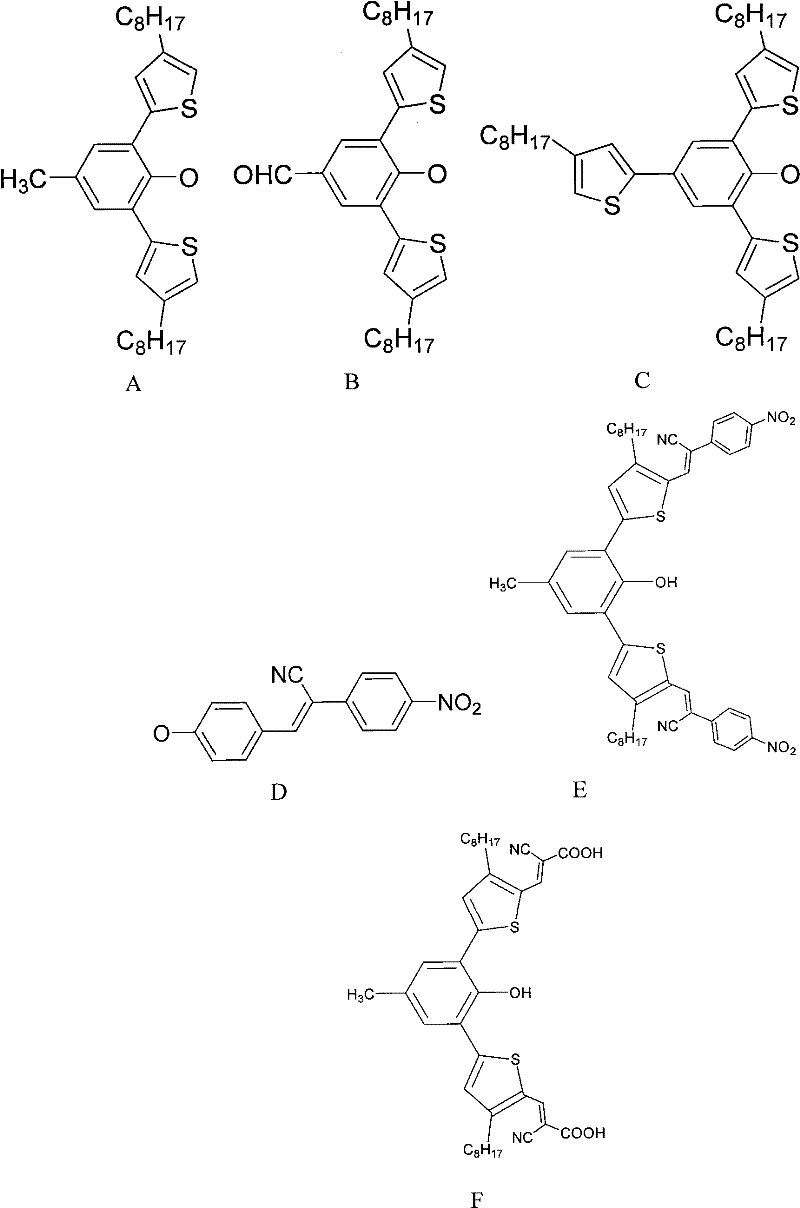
[0053] Embodiment 1: Preparation of Substituent A
[0054] Add p-cresol (2.156g, 20mmol) and 25mL of distilled water in a 100mL three-necked flask, and slowly add liquid bromine (2.256mL, 44mmol) dropwise under an ice bath. Stir at room temperature for 1 h, filter with suction, wash with water, dry, and perform column chromatography with petroleum ether as eluent to obtain 4.997 g of white solid product 2,6-dibromo-4-methylphenol, with a yield of 95%. NMR characterization data: 1 HNMR (500MHz, CDCl 3 ) δ (ppm): 7.26 (s, 2H), 5.70 (s, 1H), 2.26 (s, 3H).
[0055] Slowly add n-butyllithium (13.2mL) dropwise to 10mL fresh tetrahydrofuran (THF) dissolved with 3-octylthiophene (5.88g, 30mmol) at low temperature, lithiate for 2h, then add tributyltin chloride (10.74 g, 30mmol), reacted for 0.5h, warmed up to room temperature, reacted overnight, extracted with dichloromethane, and rotary evaporated to give a light yellow liquid tin compound of 3-octylthiophene, which was used direc...
Embodiment 2
[0057] Embodiment 2: Preparation of Substituent B
[0058] The p-cresol in Example 1 was replaced by p-Hydroxybenzaldehyde, and the feed ratio, reaction conditions and treatment methods were the same as in Example 1 to obtain substituent B with a yield of 69%. NMR characterization data: 1 H NMR (500MHz, CDCl 3 )δ(ppm): 10.01(s, 1H), 7.92(s, 2H), 6.92-6.80(s, 4H), 5.70(s, 1H), 2.69(t, 4H), 1.64-1.62(m, 4H ), 1.36-1.25 (m, 20H), 0.88 (t, 6H).
[0059] Preparation of substituent C
[0060] Phenol was used instead of p-cresol in Example 1, and the reaction conditions and treatment methods were the same as in Example 1 to obtain substituent B with a yield of 79%. NMR characterization data: 1 H NMR (500MHz, CDCl 3 )δ (ppm): 7.52 (s, 2H), 6.92-6.80 (s, 6H), 5.70 (s, 1H), 2.69 (t, 6H), 1.64-1.62 (m, 6H), 1.36-1.25 (m , 30H), 0.88(t, 9H).
Embodiment 3
[0061] Embodiment 3: Preparation of substituent D
[0062]Dissolve p-tolualdehyde (1.22 g, 10 mmol) and 4-nitrophenylacetonitrile (1.62 g, 10 mmol) in refined absolute ethanol (30 mL), dissolve 2.4 g of NaOH in 20 mL of absolute ethanol, and dropwise Added to the above solution, stirred at room temperature for 1 h, distilled under reduced pressure, cooled to obtain a dark green solid, filtered, rinsed with a large amount of water and dried to obtain substituent D with a yield of 60%. NMR characterization data: 1 H NMR (500MHz, DMSO) δ (ppm): 8.39(d, 2H), 8.08(s, 2H), 7.88(d, 2H), 7.41(d, 2H), 7.41(d, 2H), 6.82(d , 2H), 5.81 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com