(1R, 5R)-(-)-3-arylmethylene nopinone ultraviolet absorbent and preparation method thereof
A technology of arylmethylene nopinone and ultraviolet rays, which is applied in the field of ultraviolet absorbers and its preparation, can solve the problems of high price of natural camphor, great environmental pollution, long synthesis route, etc., and achieve good ultraviolet absorption effect and wide spectral range , the effect of less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of Norpinene
[0023] For the preparation method of nopinene, please refer to the literature [Research on the selective oxidation of L-β-pinene to dextrononpine, Journal of Nanjing Forestry University: Natural Science Edition, 2010, 34(2), 89-94] Method to proceed. You can also use the following methods:
[0024] Add acetone, sulfuric acid and β-pinene into a 100mL four-necked flask equipped with a stirrer, thermometer, constant pressure addition funnel and reflux condenser, cool to about 15℃ in an ice bath, and add fully ground KMnO in batches 4 , Add it in 1~1.5h. Remove the ice bath and continue the reaction at room temperature for 5-6 hours. After the reaction, filter with sand core funnel to remove MnO 2 , And then wash the solid residue with acetone. Use a rotary concentrator to recover acetone, add cyclohexane to the remainder, wash with saturated brine to neutrality, and use anhydrous Na 2 SO 4 The organic layer was dried, and cyclohexane wa...
Embodiment 2
[0025] Example 2: Preparation of (1R, 5R)-(-)-3-arylmethylene norpinene ultraviolet absorber
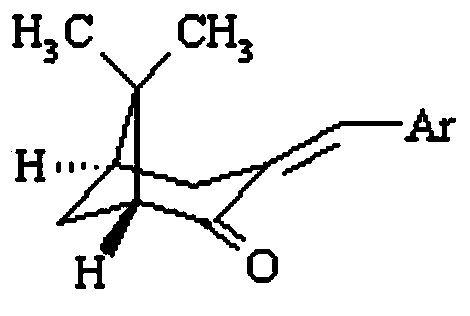
[0026] Under alkali catalysis, the (1R,5S)-(+)-nopinene prepared in Example 1 was used for hydroxylation with p-hydroxybenzaldehyde, vanillin, o-vanillin, p-chlorobenzaldehyde, and furfural. Aldehyde condensation reaction and further dehydration to obtain (1R,5R)-(-)-3-(4′-hydroxybenzylidene)nopine (1), (1R,5R)-(-)-3-(4 '-Hydroxy-3'-Methoxybenzylidene) Nopinone (2), (1R,5R)-(-)-3-(2'-Hydroxy-3'-Methoxybenzylidene) Noll Pinone(3), (1R,5R)-(-)-3-(4′-chlorobenzylidene)norpineone(4), (1R,5R)-(-)-3-(furan-2 '-Methylene) Nopinene (5).
[0027] Preparation of (1R, 5R)-(-)-3-(4'-hydroxybenzylidene)norpinene (1):
[0028] Add 1.38g (0.01mol) of norpinone, 30ml of a 10% potassium tert-butoxide solution in tert-butanol and 1.83g (0.015mol) of p-hydroxybenzaldehyde into 100mL three ports equipped with a stirrer, thermometer and reflux condenser In the flask, the reaction was refluxed for 7-8 hours ...
Embodiment 3
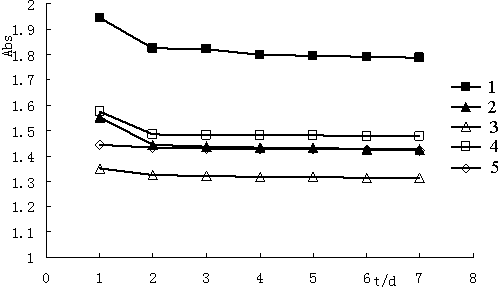
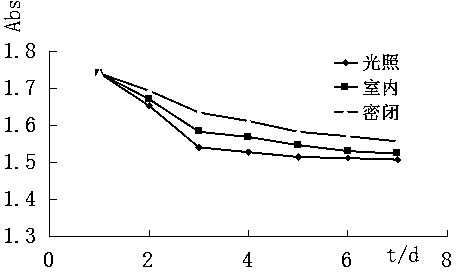
[0041] Example 3 Determination of the light absorption ability of (1R, 5R)-(-)-3-arylmethylene norpinene derivatives
[0042] The five compounds synthesized in Example 2 were dissolved in 55% ethanol and prepared into a solution with a mass fraction of about 0.0015%, and a full ultraviolet scan was performed in the range of 200-700nm to determine the absorbance range and maximum absorption wavelength. The molar absorption coefficient ε of each compound was calculated according to the following formula, and the results are shown in Table 2.
[0043] ε=A / CL
[0044] A- Absorbance
[0045] ε-molar absorption coefficient, L / mol.cm
[0046] C-The concentration of the sample, mol / L,
[0047] L-length of light path, cm
[0048] Table 2 Ultraviolet absorption range and maximum absorption wavelength of (1R, 5R)-(-)-3-arylmethylene nopinene derivatives
[0049] Compound
[0050] It can be seen from Table 2: Compounds 1-5 have strong UV absorption. The UV absorption ranges of compounds 3 and 5 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com