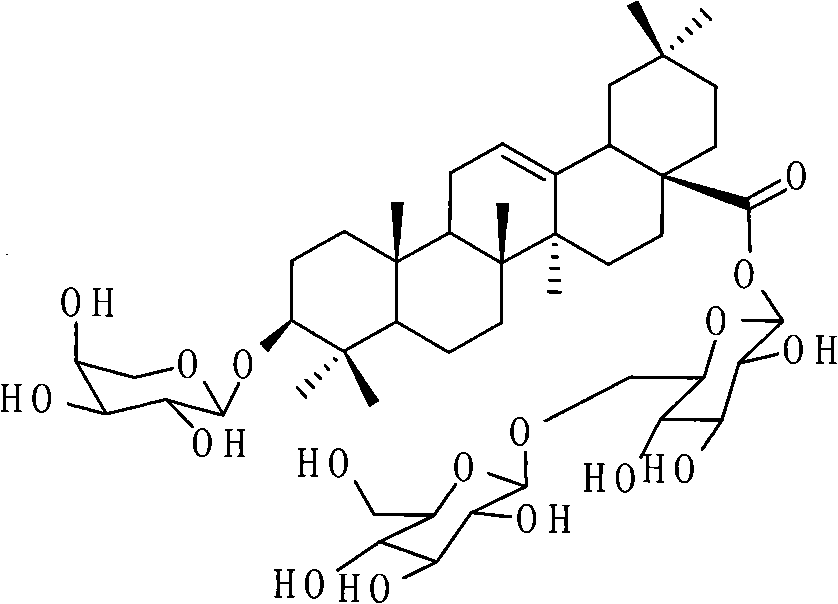
Application of akebiasaponin D in preparing medicines for treating and preventing fatty liver and related diseases
A technology of akebia saponin and fatty liver, which is applied in the fields of application, metabolic diseases, food preparation, etc., and can solve the problems of unproven curative effect, exact curative effect to be confirmed, lack of etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Separation and purification of aketoside D
[0021] According to the research results of extraction process optimization, take 5.0kg of Dipsacus chuanxiong medicinal material decoction pieces, add 70% ethanol to heat and reflux to extract twice, the amount of extraction solvent is 10 times (V / W) of the raw medicinal material each time, and the extraction time is 2 times each time. hours, combined the two extracts, recovered ethanol under reduced pressure, and obtained aketoside D transfer rate of 93%. By screening the experimental results, AB-8 macroporous adsorption resin was selected as the rough step separation method to enrich the crude product of aketoside D. The AB-8 macroporous adsorption resin column was 10cm in diameter x 65cm in length, and the weight ratio of resin dosage to medicinal material It is 1:1. Dilute the above-mentioned concentrated drug solution with water to a solid content of 8%, load the sample, wash with 3 column volumes of water, r...
Embodiment 2
[0022] Example 2 Preparation of aketoside D
[0023] Test 1. Preparation of Aketoside D Injection
[0024] Prescription 1: Akebia saponin D 1000mg, solubilizer F68 20mg, cosolvent propylene glycol 60mg, antioxidant sodium sulfite 30mg. Adjust the isotonicity with anhydrous glucose, adjust the pH value to 7 with buffer salt, and add redistilled water to 150mL.
[0025] Prescription 2: Akebia saponin D 1000mg, solubilizer F68 25mg, HP-β-cyclodextrin 2000mg, antioxidant sodium sulfite 50mg. Dissolve the prescribed amount of HP-β-cyclodextrin in 40mL of ethanol at 600r.min -1 Add aketoside D powder under stirring, continue to stir for 2 hours, and set aside; take sodium bisulfite and F68 and add 50mL aqueous solution to dissolve, and inject aketoside D's HP-β-cyclodextrin ethanol solution under stirring until aketoside D was completely dissolved, then pre-frozen at -60°C, and dried in vacuum to obtain an off-white loose freeze-dried powder.
[0026] Test 2. Preparation of Aket...
Embodiment 3
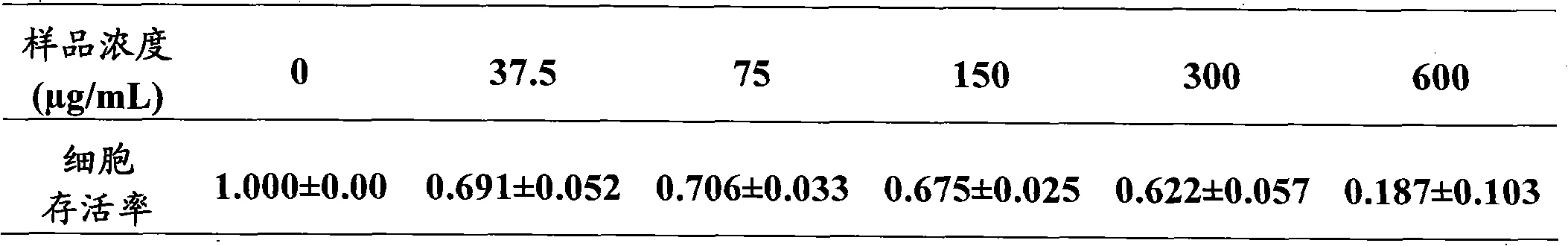
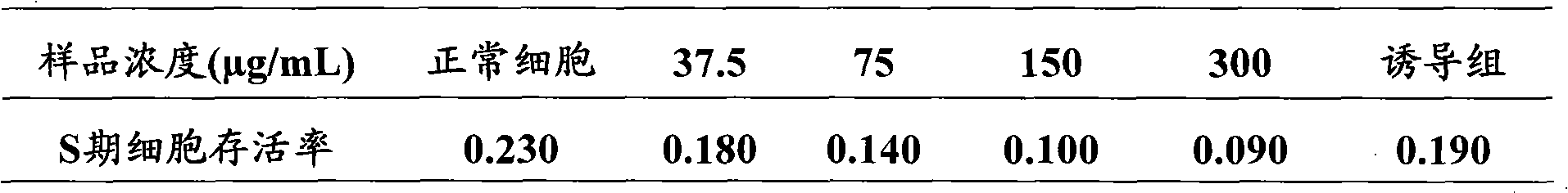
[0029] Example 3 Aketoside D inhibits the growth and proliferation of adipocytes
[0030] Material: Akebia saponin D content 98% (prepared by the Pharmacy Department of the Second Artillery General Hospital, batch number: 20090926; dissolved in sterilized distilled water, refrigerated and stored at 4°C for later use, when dosing, use DMEM / Ham's F12 containing 10% fetal bovine serum culture medium diluted to the desired concentration). Mouse preadipocyte 3T3-L1 (Cell Center, Chinese Academy of Medical Sciences). DMEM / Ham's F12 medium (Gibco); fetal calf serum (FCS, Gibco); newborn calf serum (NCS, Gibco); dimethyl sulfoxide (DMSO, Sigma); tetramethylazozolium salt (MTT, Sigma ); propidium iodide (PI, Sigma); TritonX-100 (Sigma); trypsin (AMRESCO); 3-isobutyl-1-methylxanthine (IBMX, Sigma); dexamethasone (Dex, Sigma ); Insulin (Insulin, Sigma). LS-1F ultra-clean bench (Shanghai Thorpe Instruments); 4950E CO2 cell incubator (USA, NUNR); AU640 inverted microscope (Olympus); 550...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com