Composition for solid electrolyte and solar cell using same
一种固体电解质、组合物的技术,应用在电解电容器、分散在不导电无机材料中的导电材料、电路等方向,能够解决电解质粘度变高、电池寿命劣化、离子扩散能力变差等问题,达到有效电荷移动、充分电荷寿命的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
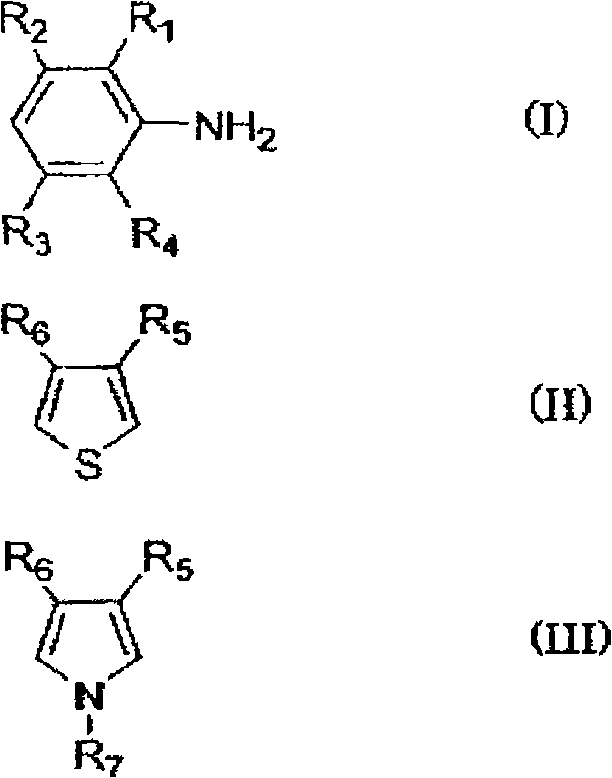
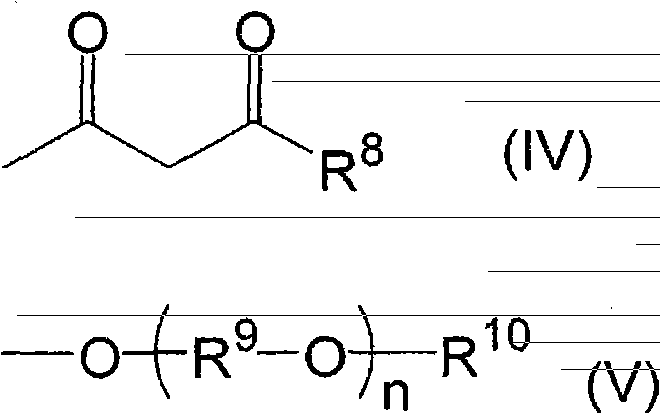
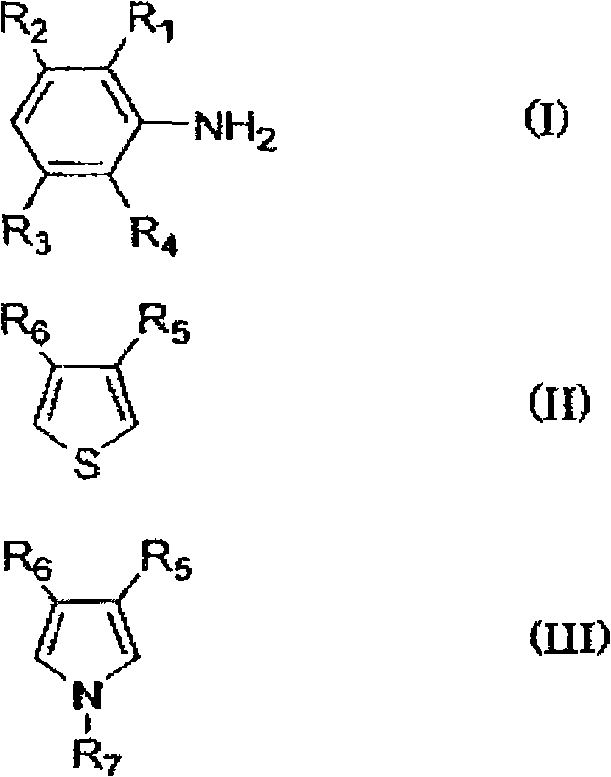
[0147] In the production of the π-conjugated polymer (β), for example, first, if necessary, an electrolytic matrix solvent such as ion-exchanged water is acidified, and an emulsifier such as p-toluenesulfonic acid is added thereto. Then, monomers (I) to (III) are added thereto, and an oxidizing agent is added thereto to be oxidatively polymerized.
[0148] In the above reaction, examples of the acidic component used to make the electrolytic matrix solvent acidic include hydrochloric acid, sulfuric acid, perchloric acid, periodic acid, iron(III) chloride, and iron(III) sulfate. What is necessary is just to use about 0.5-4.0 mol of acidic components with respect to 1 mol of total amounts of the monomers (I)-(III) used about the quantity of an acidic component.
[0149] In addition, as the oxidizing agent used for the reaction, although it needs to be adjusted appropriately according to the redox potential of the aromatic compound (monomer) forming the π-conjugated polymer (β), e...
Embodiment
[0265] Although the conductive polymer composition of the present invention was specifically described by showing examples, the present invention is not limited to these examples.
[0266] In the examples, the molecular weight was measured by the following method.
[0267]
[0268] Measurement was carried out by GPC under the following conditions.
[0269] Device name: HLC-8120 (manufactured by Tosoh Co., Ltd.)
[0270] Column: GF-1G7B+GF-510HQ (Asahipak: registered trademark, manufactured by Showa Denko Co., Ltd.)
[0271] Standard material: polystyrene and sodium polystyrene sulfonate
[0272] Sample concentration: 1.0mg / ml
[0273] Eluent: 50 mmol lithium chloride aqueous solution / CH3CN=60 / 40wt
[0274] Flow rate: 0.6ml / min
[0275] Column temperature: 30°C
[0276] Detector: UV254nm
preparation example 1-1
[0278] With a mixer, nitrogen inlet tube, reflux condenser, inlet and thermometer, capacity 1000cm 3 In a four-necked flask, drop 24.3g of sodium methacrylate 2-ethanesulfonate (MS-2N) (25% by mole), 25.7g of acetoacetoxyethyl methacrylate (AAEM) (25% by mole) 15%), 42.8g of benzyl methacrylate (BzMA) (30% by mole), 48.2g of 2-ethylhexyl methacrylate (2EHMA) (30% by mole), 150g of ion-exchanged water, 300 g of isopropyl alcohol (IPA). While introducing nitrogen into the flask, the temperature of the mixture in the flask was raised to 70°C. Then, 0.7 g of azobisisobutyronitrile was charged into the flask, and a polymer solution (A-1) containing a polymer compound (A) was obtained by performing a polymerization reaction at 70° C. for 18 hours.
[0279] Move the whole amount of the resulting polymer solution (A-1) to 2000cm 3 500 g of hexane was added while being stirred by a stirrer, and then left to stand for 1 hour to remove the oil layer containing impurities. The solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com