Titanium niobate composite material, preparation method thereof, and cathode and battery containing the same
A composite material, titanium niobate technology, applied in battery electrodes, lithium batteries, non-aqueous electrolyte batteries, etc., can solve problems such as large structural changes, irreversible capacity, and low conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
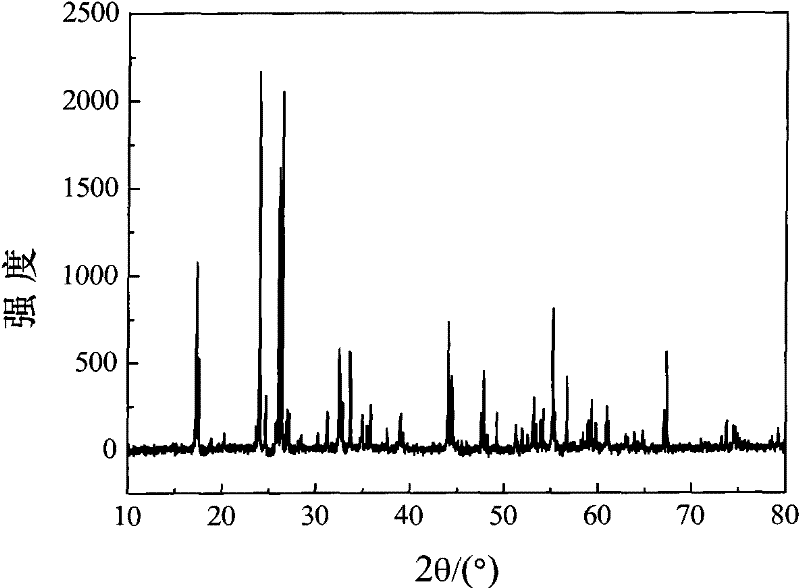
[0065] According to TiNb 2 o 7 The ratio of each element in the high-purity TiO 2 Powder and Nb 2 o 5 Put the powder and an appropriate amount of alcohol into the mixing tank, then ball mill for 10 hours, dry at 50-70°C, then raise the temperature to 1200-1400°C at a rate of 5°C / min in the air atmosphere, and keep it warm for 24 hours , followed by cooling to room temperature, yielding pure-phase TiNb 2 o 7 sample. figure 1 gives the pure phase TiNb 2 o 7 X-ray diffraction pattern of the sample. Experiments show that: Ti and Nb elements in TiNb 2 o 7 The interoccupancy in will affect its XRD sealing strength ratio, and this interoccupancy is easy to occur in the process of preparing materials.
Embodiment 2
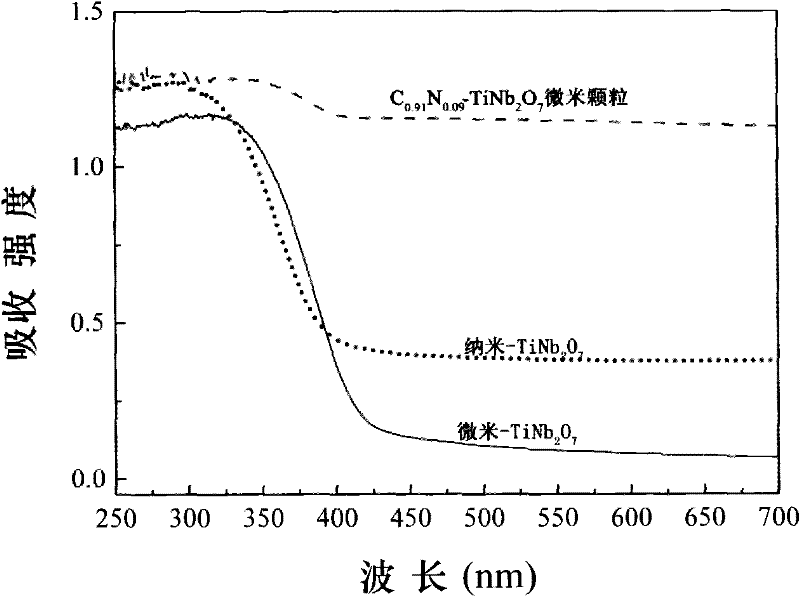
[0067] To the pure phase TiNb that embodiment 1 prepares 2 o 7 Materials do UV-Vis absorption spectrum, the results are as follows figure 2 shown by figure 2 It can be seen that TiNb 2 o 7 The absorption edge of the material is about 420nm, and its bandgap can be obtained by transformation at 2.95eV. Combined with first-principle calculations, it can be known that it is a material with an indirect bandgap, which indicates that it has potential applications in the short-wavelength band of visible light absorption, and can be used as A material that absorbs light, or is used in photovoltaic cells. Simultaneous experiments and calculations demonstrate its potential application in electrochromism.
[0068] The pure phase TiNb prepared by embodiment 1 2 o 7 Materials and Ionic Liquids[EMIm][N(CN) 2 ] After uniform mixing; in a tube furnace, in an argon atmosphere, the resulting mixture is heated from room temperature to between 500 and 700 °C at a rate of 5 °C / min, and ke...
Embodiment 3
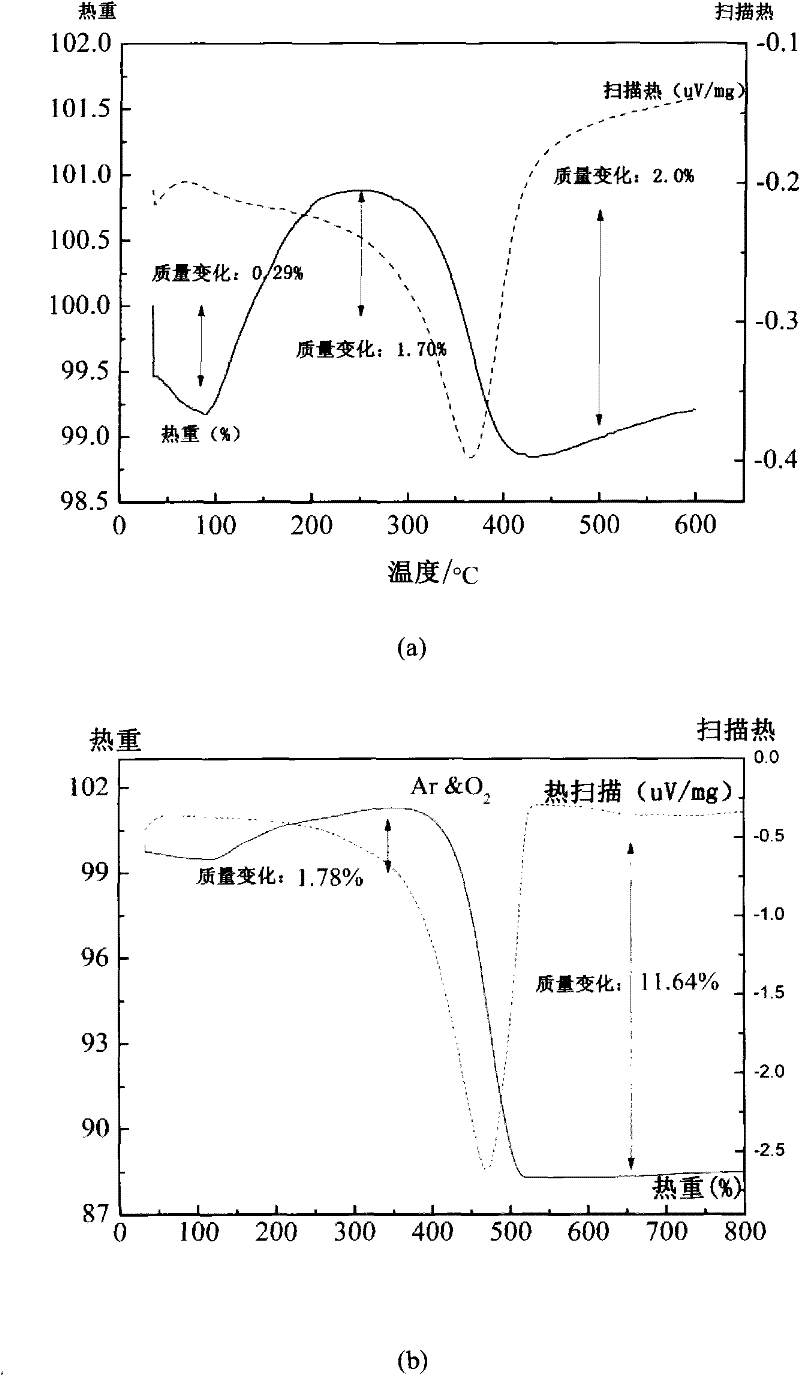
[0070] The pure phase TiNb prepared by embodiment 1 2 o 7 The sample was subjected to thermogravimetric differential heat experiment in Ar atmosphere, and the results were as follows: image 3 (a) shown. In the range of 100°C to 600°C, there is an obvious oxygen absorption and release phenomenon, which indicates that TiNb 2 o 7 May be a potential oxygen storage material.
[0071] Composite material C prepared in embodiment 2 0.91 N 0.09 -TiNb 2 o 7 in Ar and O 2 In the thermogravimetric differential heat experiment in a mixed atmosphere, there is an obvious weight loss phenomenon, which is attributed to the oxidation of boron carbon or carbon nitrogen or boron carbon nitrogen compounds, and then detached from the surface, such as image 3 (b) shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com