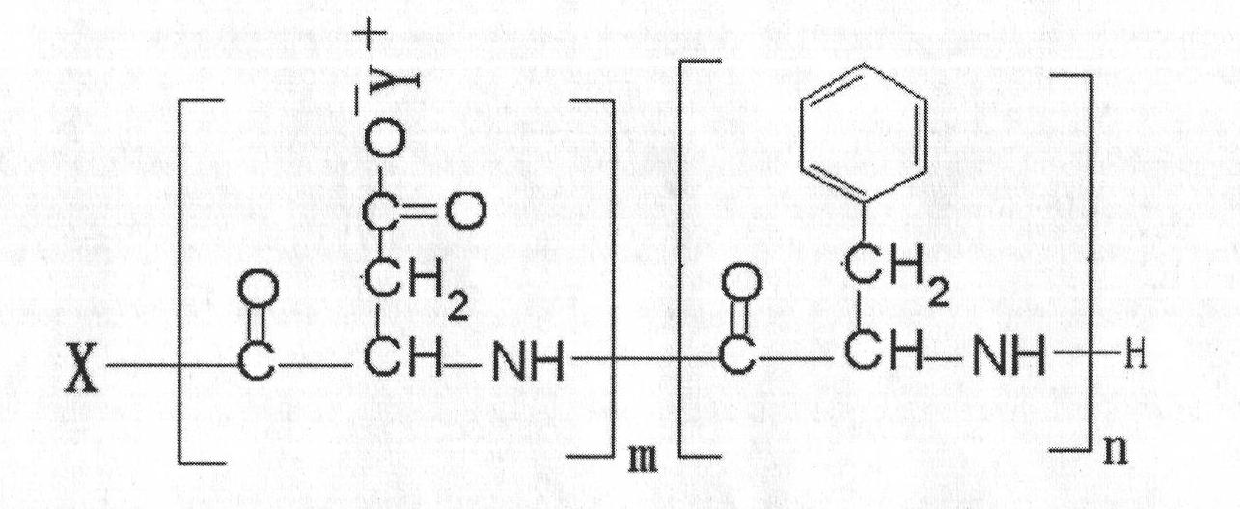
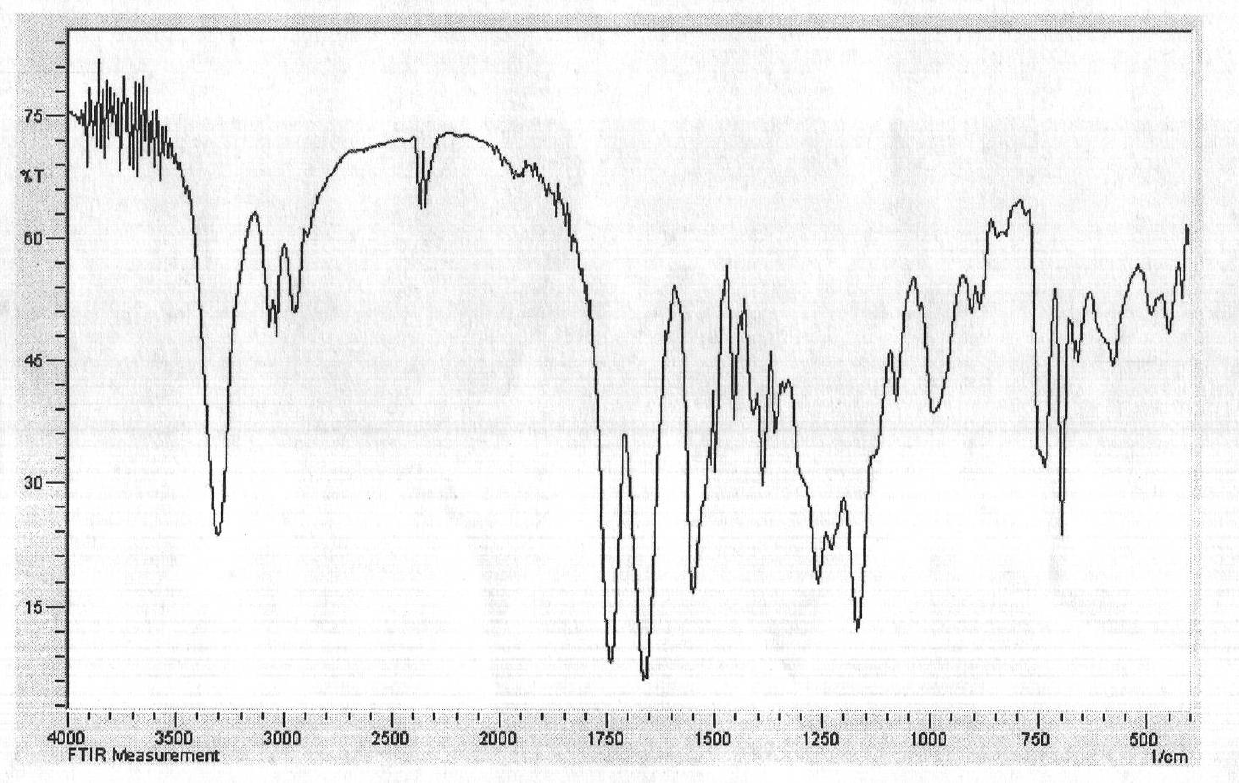
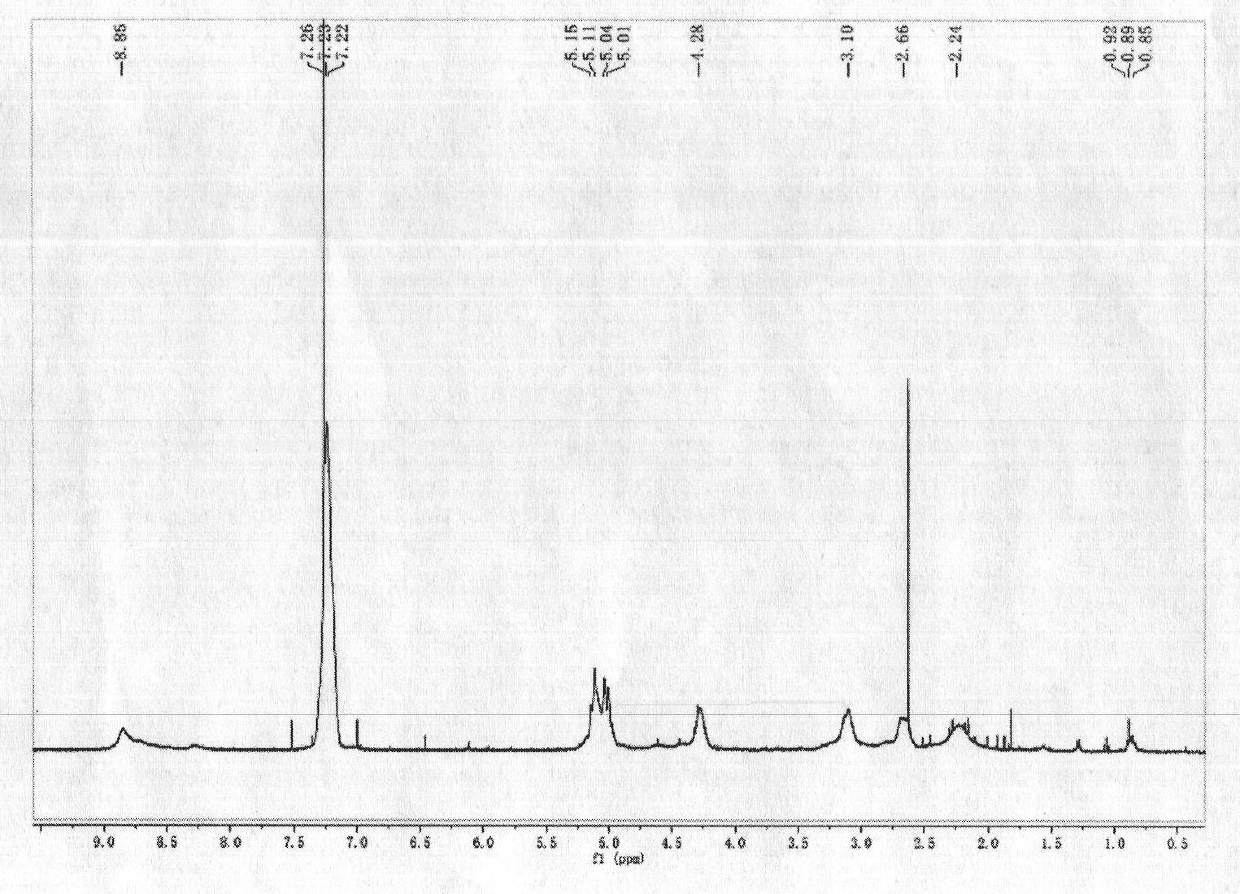
Amphipathic amino acid block copolymer and preparation method and application thereof
A block copolymer and amphiphilic technology, which is applied in the biodegradable amphiphilic amino acid block copolymer as a drug carrier, in the field of preparation of the carrier, the technology is mature, the tumor targeting is increased, and the nanomicelle is stable. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Preparation of L-aspartic acid-β benzyl ester internal anhydride and L-phenylalanine internal anhydride
[0063]References (Prompruk K, Govender T, Zhang S, et al.Synthesis of a novel PEG-block-poly(aspartic acid-statphenylalanine) copolymer shows potential for formation of a micellar drug carrier[J].International Journal ofPharmaceutics, 2005, 297(1-2):242-253.) proceed. Among them, L-aspartic acid-β benzyl ester and L-phenylalanine were purchased from Shanghai Hanhong Chemical Co., Ltd., and triphosgene was purchased from Shanghai Jingchun Reagent Co., Ltd.
[0064] (1) Preparation of L-phenylalanine internal acid anhydride: Add 10g of L-phenylalanine to 100ml of anhydrous tetrahydrofuran, stir magnetically, heat up to 50°C, then add 15g of triphosgene, the suspension becomes clear, and fill with nitrogen for 30min. To remove hydrogen chloride gas and remaining phosgene generated by the reaction, the reaction solution was concentrated, poured into exces...
Embodiment 2
[0070] Embodiment 2: the preparation of amphiphilic amino acid block copolymer
[0071] Dissolve 10g of L-aspartic acid-benzyl ester cyclic acid anhydride in 20mL of dimethylformamide, add 8mg of n-propylamine and stir at room temperature for 72h, add absolute ethanol to precipitate, collect the precipitate, weigh 1g of dimethylformamide after vacuum drying Add 0.95 g of L-phenylalanine cyclic anhydride, continue to stir at room temperature for 72 hours, add ethanol to precipitate, collect the precipitate, add 2M NaOH 5 mL after vacuum drying, stir at room temperature for 4 hours, dialyze with distilled water for 24 hours, freeze-dry Instantly. Yield 19.7%. 1 HNMR determination, by calculating the chemical shift peak area of the methine proton at the α-position of the aspartic acid segment, the chemical shift peak area of the methine proton at the α-position of the phenylalanine segment and the chemical shift peak of the methyl proton in the terminal propyl group The are...
Embodiment 3
[0074] Embodiment 3: the preparation of amphiphilic amino acid block copolymer
[0075] Dissolve 10g of L-aspartic acid-β benzyl ester cyclic anhydride in 20mL of dimethylformamide, add 500mg of n-butylamine and stir at room temperature for 12h, add absolute ethanol to precipitate, collect the precipitate, dry it in vacuum, weigh 1g with two Dissolve 10mL of methylformamide, add 1.2g of L-phenylalanine internal anhydride and continue stirring at room temperature for 12h, add absolute ethanol for precipitation, collect the precipitate, dry it in vacuum, add 0.5M NaOH 5mL, stir at room temperature for 1h, dialyze with distilled water for 24h, It can be obtained by freeze-drying. Yield 45.6%. 1 HNMR determination, by calculating the chemical shift peak area of the methine proton at the α-position of the aspartic acid segment, the chemical shift peak area of the methine proton at the α-position of the phenylalanine segment and the chemical shift peak of the methyl proton in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com