Preparation method of 2-chloro-3-aminopyridine
A technology of aminopyridine and nitropyridine, which is applied in the field of preparation of 2-chloro-3-aminopyridine, can solve the problems of high price, cumbersome operation, and difficult operation, so as to reduce production cost, increase reaction yield, and simplify purification The effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
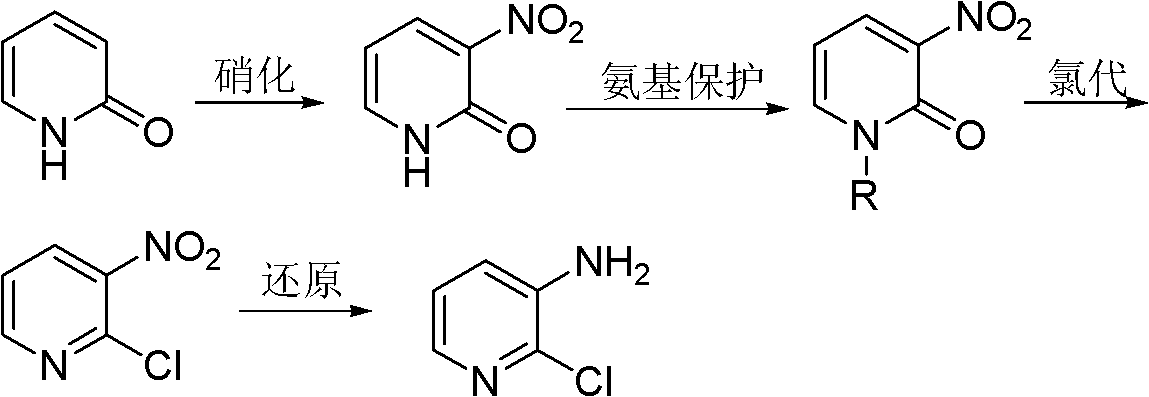
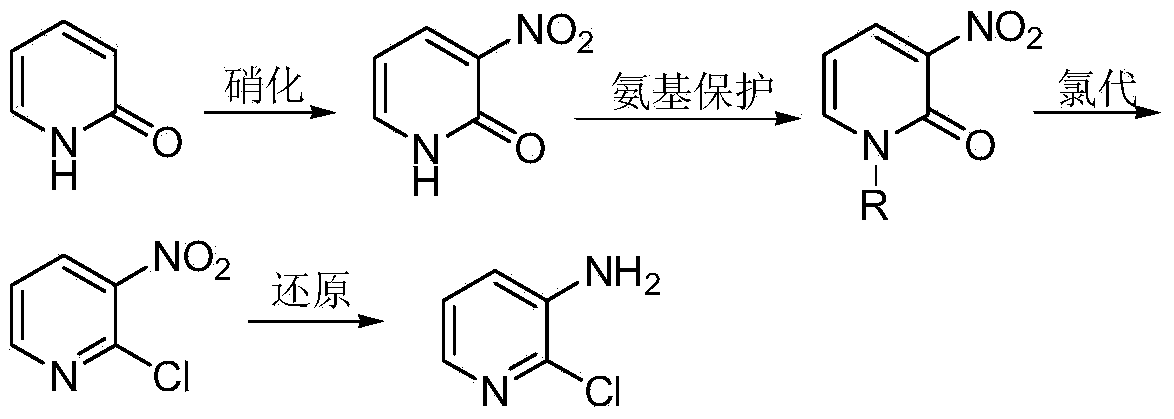
[0036] Embodiment 1: the preparation of N-alkyl-3-nitro-2-pyridone
[0037] 2-Pyridone (100g, 1.05mol) was added to a four-necked flask filled with concentrated sulfuric acid (200mL), and then a mixture of fuming nitric acid (67mL) and concentrated sulfuric acid (100mL) was added to the above-mentioned four-necked flask . The temperature of the whole process was controlled below 40°C, and stirred for 2h under this condition. Then, ice (900 g) and 40% NaOH (150 mL) solution were added to the reaction liquid. The mixture was extracted with toluene (1000 mL×3), the organic phases were combined and the precipitated crystals were filtered off. Concentrate and cool the filtrate to precipitate 3-nitro-2-pyridone; recrystallize with methanol to remove a small amount of 5-nitro-2-pyridone with relatively high solubility to obtain 3-nitro-2-pyridone Ketone 31.6g, m.p.224~225℃.
[0038] 3-nitro-2-pyridone (28g, 0.2mol) was dissolved in DMF (20mL), and then 10% KOH (16.8g, 0.3mol) aqu...
Embodiment 2
[0039] Embodiment 2: the preparation of 3-nitro-2-chloropyridine
[0040] Add N-methyl-3-nitro-2-pyridone (20 g, 0.13 mol) into a four-necked flask equipped with a reflux condenser, a thermometer and a stirring blade, and heat to 130° C. to dissolve it; then slowly Triphosgene (16.2 g, 0.054 mol) was added and the reaction mass was liquid. After the chlorination reaction is finished, add an appropriate amount of 40% NaOH aqueous solution (w / w) for treatment, adjust the pH to be weakly alkaline, and then carry out steam distillation to obtain 19.25 g of 2-chloro-3-nitropyridine with a yield of 93% , the content is 98.6%.
Embodiment 3
[0041] Embodiment 3: Preparation of 2-chloro-3-aminopyridine
[0042] In a nitrogen atmosphere, the TiCl 4 (2 mL, 0.018 mol) was slowly added into anhydrous tetrahydrofuran (50 mL) which was continuously stirred, and the temperature was controlled at 0°C. Further Mg (0.95 g, 0.04 mol) was added to the obtained yellow suspension, followed by reflux for 3 h to obtain a black suspension.
[0043] 2-Chloro-3-nitropyridine (16 g, 0.1 mol) was added portionwise to the above suspension at 0°C. The reaction mixture was stirred at room temperature for 1 h, then water (500 mL) and 25% aqueous ammonia solution (500 mL) were added. The mixture was extracted with ether (500 mL×5). MgSO for organic phase 4 After drying, it was evaporated to obtain 12.7 g of 2-chloro-3-aminopyridine with a yield of 98% and a content of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com