Method for full chemical synthesis of fibrauretin anti-bacterial anti-inflammatory medicine
A technology of medicine patellogenin, antibacterial and anti-inflammatory, applied in the direction of organic chemistry, etc., can solve the problems of high equipment requirements, low yield, and complicated operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
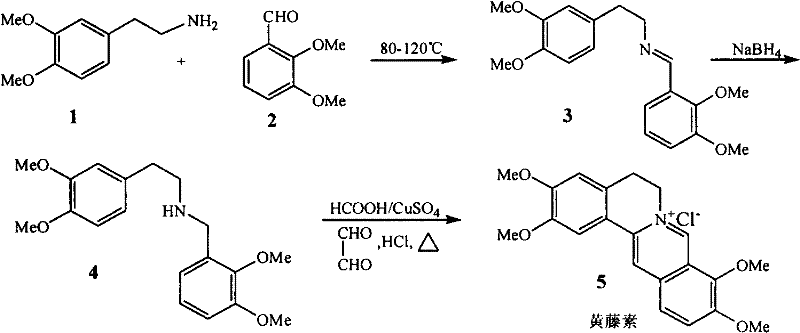
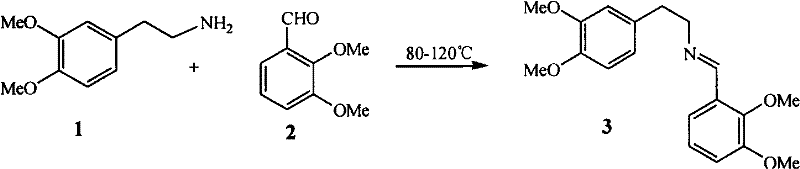
[0027] (1) Synthesis of N-(3,4-dimethoxyethylphenyl)-C-(2,3-dimethoxyphenyl)imine 3
[0028] Add 3,4-dimethoxyphenethylamine and 2,3-dimethoxybenzaldehyde to the reaction bottle, and raise the temperature to 120°C for 4 hours under stirring. The product is not purified, and 3.01g of viscous reddish brown is obtained. liquid (3).
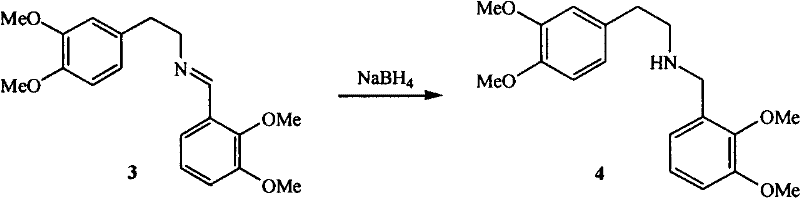
[0029] (2) Synthesis of N-(2,3-dimethoxybenzyl)-(3,4-dimethoxy)phenethylamine 4
[0030] After 3 was cooled to room temperature, methanol was added to dissolve, and sodium borohydride was added in portions under stirring at room temperature, and reflux was continued for 2 hours after addition, methanol was recovered, the residue was dissolved in water, extracted with ethyl acetate, anhydrous Na 2 SO 4 After drying, the solvent was recovered after filtration to obtain 2.88 g of yellow viscous liquid 4, and the pH value of the obtained product was adjusted with concentrated hydrochloric acid for later use.
[0031] Synthesis of target product palmat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com