Nitrogen oxides luminescent material and preparation method thereof and lighting source made of same
A technology of nitrogen oxides and luminescent materials, applied in the semiconductor field, can solve the problems of polluting the environment, easy decomposition, exhaust gas discharge, etc., and achieve the effect of high energy conversion and high brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Sr 0.90 Li 0.05 Si 4 AlN 7 :Ce 0.05 Preparation examples of luminescent materials
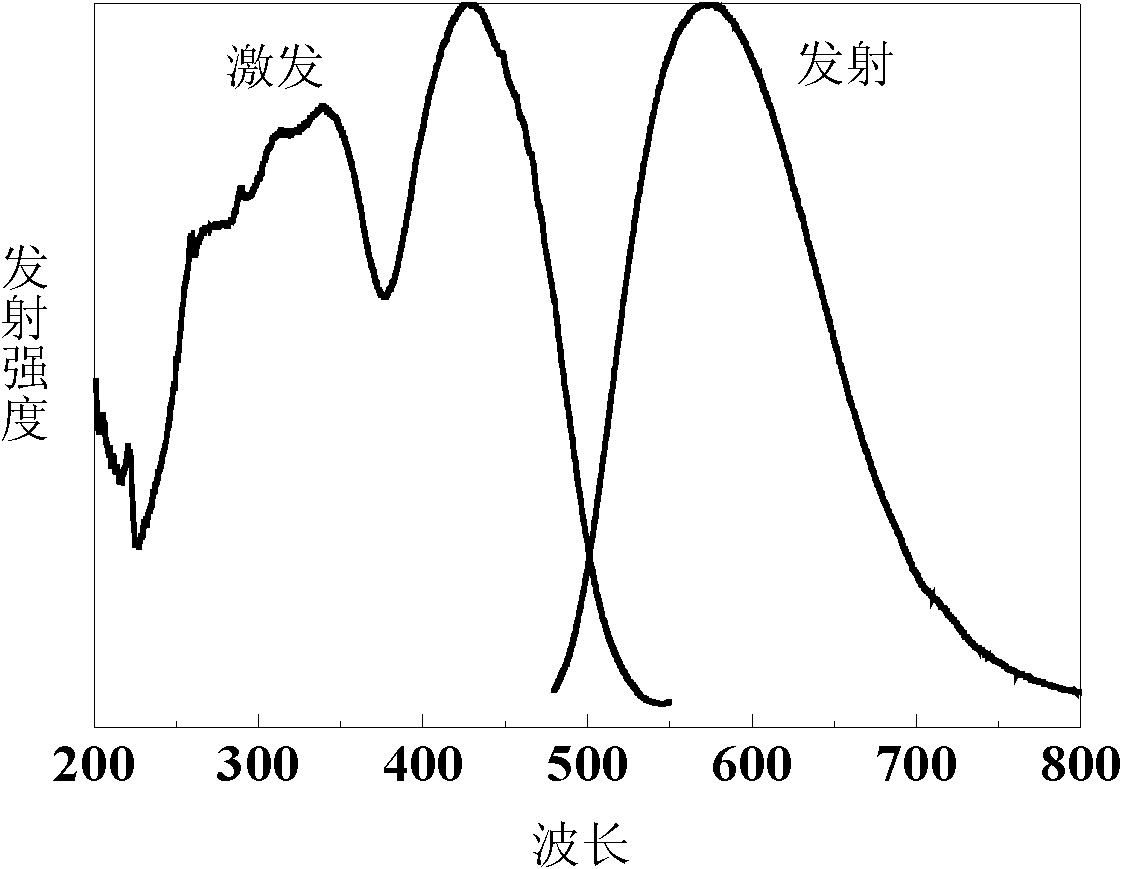
[0045] Weigh Sr according to the above composition 3 N 2 (27.0746g), Li 3 N (0.1803 g), Si 3 N 4 (57.6933g), CeN (2.3798g) and AlN (12.6719g), after being mixed and ground uniformly in a glove box filled with argon, put in a boron nitride crucible and fired in an air pressure furnace, and pass 0.3MPa N 2 ,Hold at 1700°C for 4 hours, and the powder obtained is ground and then calcined at high temperature under the same conditions to promote the growth of crystal grains. The obtained luminescent material is crushed, washed with hydrochloric acid to remove impurities, and dried to obtain 100 g of the yellow luminescent material of the present invention. See the emission spectrum and excitation spectrum figure 1 . From figure 1 It can be found that the emission spectrum of the luminescent material is relatively wide, the half-height width of the spectrum is about 130nm, and the main ...
Embodiment 9
[0046] Example 9: Sr 0.90 Li 0.05 Si 3.85 Al 1.15 O 0.15 N 6.85 :Ce 0.05 Preparation examples of luminescent materials
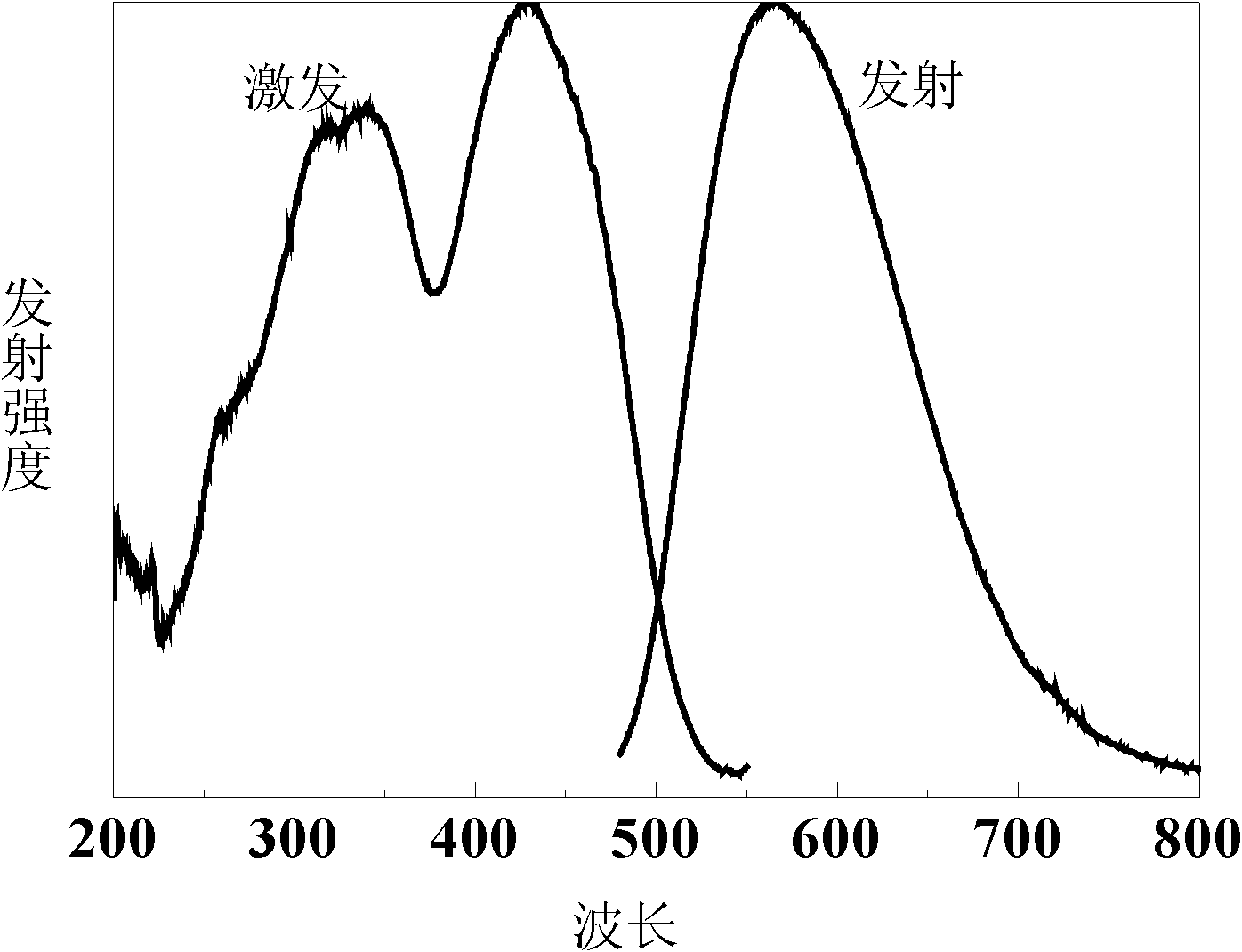
[0047] Weigh Sr according to the above composition 3 N 2 (27.0204g), Li 3 N (0.1799 g), Si 3 N 4 (55.4185g), Ce 2 O 3 (2.5293g), Al 2 O 3 (1.5731g) and AlN (13.2788g), after being mixed evenly in a glove box filled with argon, put into a boron nitride crucible and fired in an air pressure furnace, and pass 0.3MPa N 2 , With 0.1gSrF 2 As a flux, it is kept at 1700°C for 4 hours, and the obtained powder is ground and then calcined at a high temperature under the same conditions to promote the growth of crystal grains. The obtained luminescent material is crushed, washed with hydrochloric acid to remove impurities, and dried to obtain 100 g of the yellow luminescent material of the present invention. See the emission spectrum and excitation spectrum figure 2 . From figure 2 It can be found that the emission spectrum of the luminescent material is relatively wide...
Embodiment 2-8 and 10-16
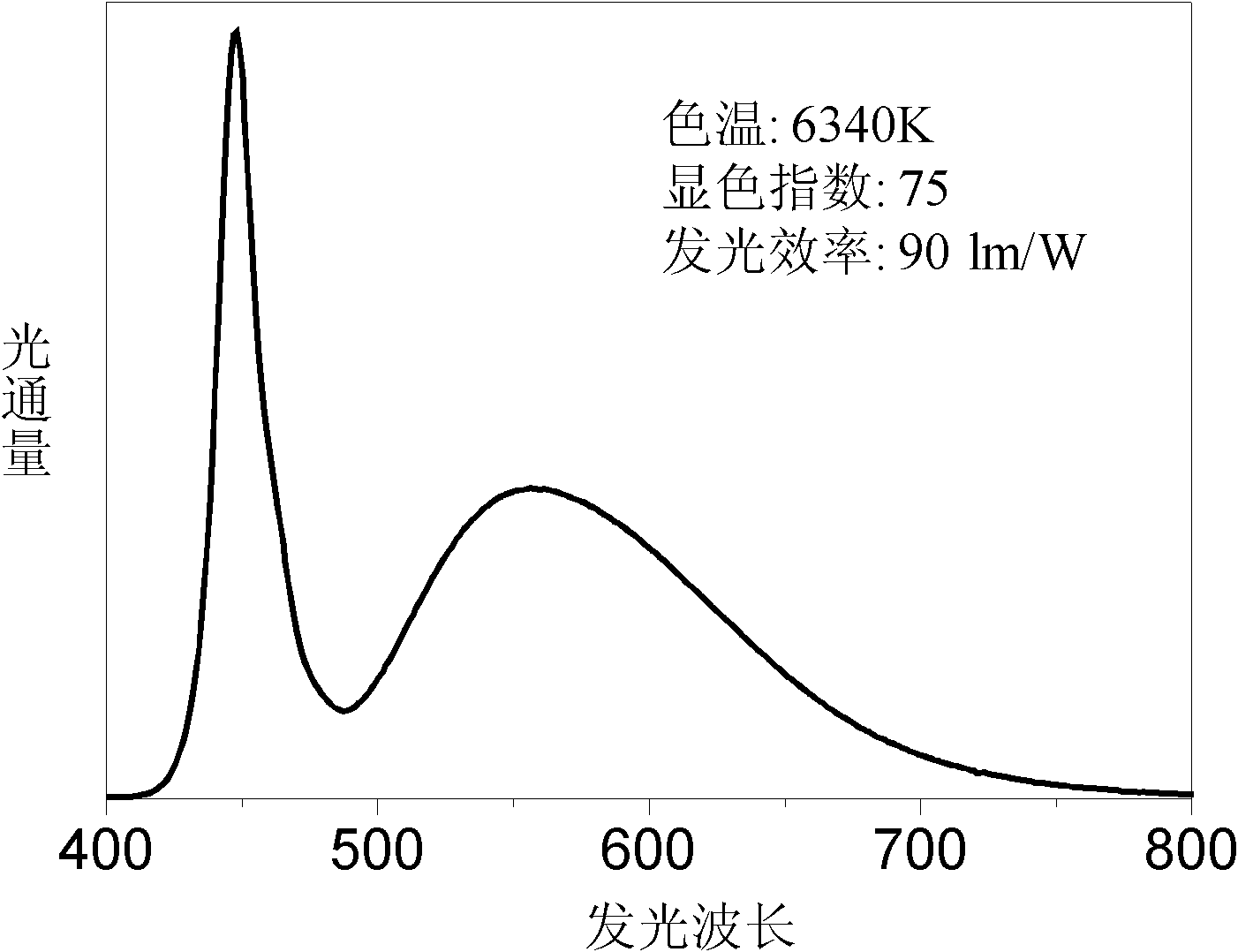
[0049] The preparation process of the above embodiment is the same as that of embodiment 1 or embodiment 9, in which Ce halide such as CeCl can also be used 3 Or nitrates such as Ce(NO 3 ) 3 The reaction flux used is chloride or fluoride such as Sr, Ca, Ba, Li, etc. The luminous intensity of the luminescent material obtained is shown in Table 1. The maximum emission wavelength of these luminescent materials is mostly in the yellow light region, and can be excited by blue and ultraviolet light, and can replace YAG phosphors to prepare white light LEDs.
[0050] Table 1 Chemical formula and luminescence characteristics of Examples 1-18 (excitation wavelength is 450nm)
[0051] Example
Main emission peak nm
Relative Strength%
[0052] 1
Sr 0.90 Li 0.05 Si 4 AlN 7 :Ce 0.05
573
100
2
Sr 0.80 Li 0.10 Si 4 AlN 7 :Ce 0.10
576
94
3
Sr 0.85 Ca 0.05 Li 0.05 Si 4 AlN 7 :Ce 0.05
580
85
4
Sr 0.85 Ba 0.05 Li 0.05 Si 4 AlN 7 :Ce 0.05
568
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com