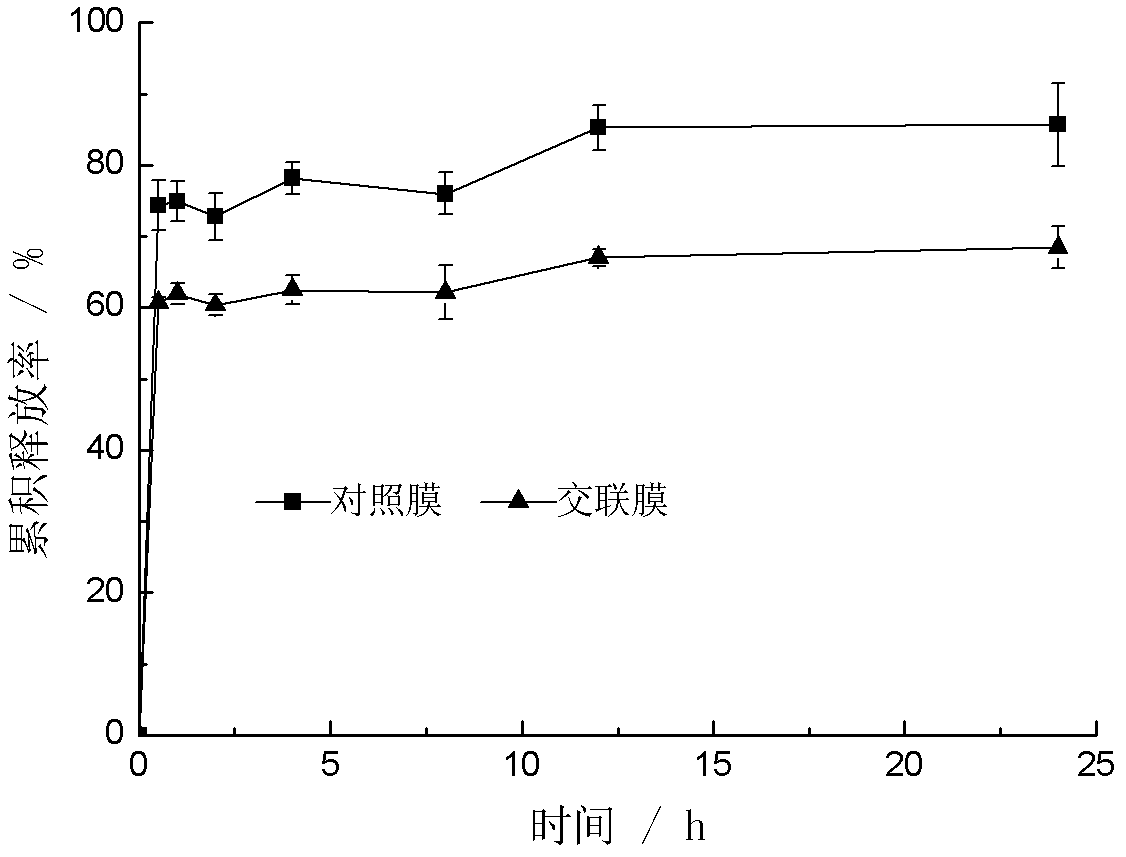
Preparation method and application of drug-carrying keratin film
A keratin and drug-loading technology is applied in the field of preparation of drug-loaded keratin membranes, which can solve the problems of high brittleness, toxic and side effects of chemical cross-linking agents, large pollution, etc., and achieves improved tensile strength, broad application prospects, and environmental protection. The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation method of embodiment 1 drug-loaded keratin film
[0016] After the wool fabric is washed and dried, it is degreased with a 4:1 mixture of chloroform and methanol, and the degreased wool is dissolved in 0.4mol / L sodium bisulfite, 7mol / L urea and 0.15mol / L dodecylsulfuric acid In the sodium mixed solution, the dissolution temperature is 70°C, the dissolution time is 4 hours, and the pH value is kept between 6-7. The resulting solution is dialyzed in distilled water for 2 days to obtain an aqueous solution of keratin. Concentration concentration (w / v) to 10%; the weight ratio of keratin solution and plasticizer glycerin is 1.5%, adding DTT content is 10mmol / L, adjusting the pH value to 7.5 with NaOH, adding 1% medicine, and adding 30U glutamine per gram of keratin Amide transaminase, stirred evenly, and kept at 37°C for 8 hours, degassed, poured on a glass plate lined with polyethylene film, dried naturally at room temperature for 24 hours, and peeled off t...
Embodiment 2
[0017] The preparation method of embodiment 2 drug-loaded keratin film
[0018] After the wool fabric is washed and dried, it is degreased with a 4:1 mixture of chloroform and methanol, and the degreased wool is dissolved in 0.6mol / L sodium bisulfite, 9mol / L urea and 0.05mol / L dodecylsulfuric acid In the sodium mixed solution, the dissolution temperature is 50°C, the dissolution time is 7 hours, and the pH value is kept between 6-7. The resulting solution is dialyzed in distilled water for 2 days to obtain an aqueous solution of keratin. Concentration concentration (w / v) to 4%; the keratin solution and the plasticizer glycerin are 0.5% by weight, the content of DTT is added to 20mmol / L, the pH value is adjusted to 7 with NaOH, 2% of the drug is added, and 30U glutamine is added per gram of keratin Amide transaminase, stirred evenly, and kept at 37°C for 14 hours, degassed, poured on a glass plate lined with polyethylene film, dried naturally at room temperature for 24 hours, a...
Embodiment 3
[0019] The preparation method of embodiment 3 drug-loaded keratin film
[0020] After the wool fabric is washed and dried, it is degreased with a 4:1 mixture of chloroform and methanol, and the degreased wool is dissolved in 0.6mol / L sodium bisulfite, 8mol / L urea and 0.06mol / L dodecylsulfuric acid In the sodium mixed solution, the dissolution temperature is 65°C, the dissolution time is 5 hours, and the pH value is kept between 6-7. The resulting solution is dialyzed in distilled water for 3 days to obtain an aqueous solution of keratin. Concentration concentration (w / v) to 6%; the keratin solution and the plasticizer glycerin are 1.5% by weight, the pH value is adjusted to 8.5 with NaOH, 3% of the medicine is added, 30U transglutaminase is added per gram of keratin, stirred evenly, and Keep it warm at 55°C for 3 hours, pour it on a glass plate lined with polyethylene film after degassing, dry naturally at room temperature for 24 hours, and peel off the film to obtain a drug-l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com