Method and device for hydrogen-circulating electrolysis and application of the method and device in production of aluminum oxide
An electrolysis method and a technology of hydrogen circulation, applied in the direction of electrolysis components, electrolysis process, cells, etc., can solve the problem of high energy consumption in the electrolysis of carbon fraction mother liquor, and achieve the effect of reducing energy consumption, reducing power consumption, and reducing power consumption indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
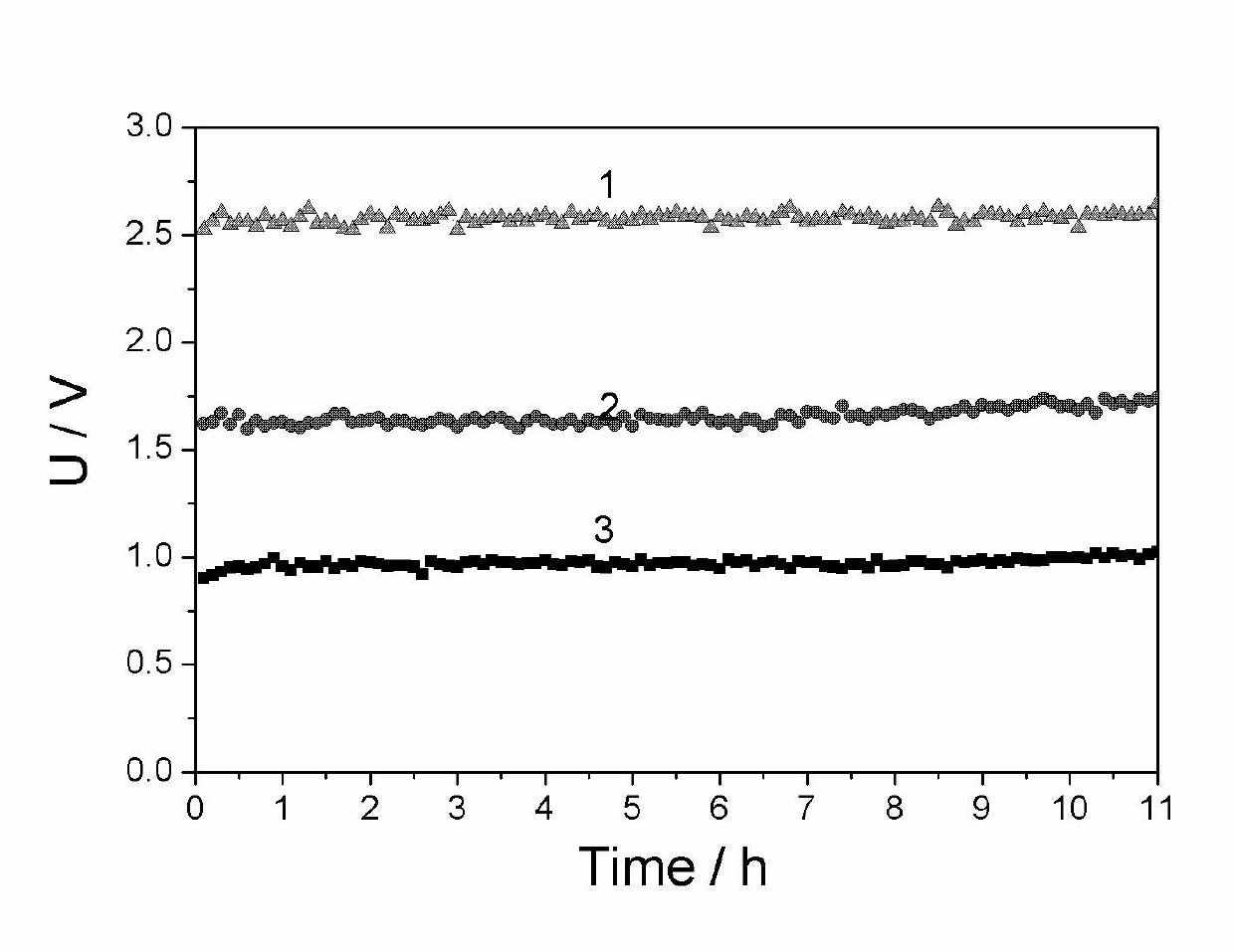
[0030] Example 1 (comparative example, using the electrolysis method of hydrogen evolution at the cathode and oxygen evolution at the anode)
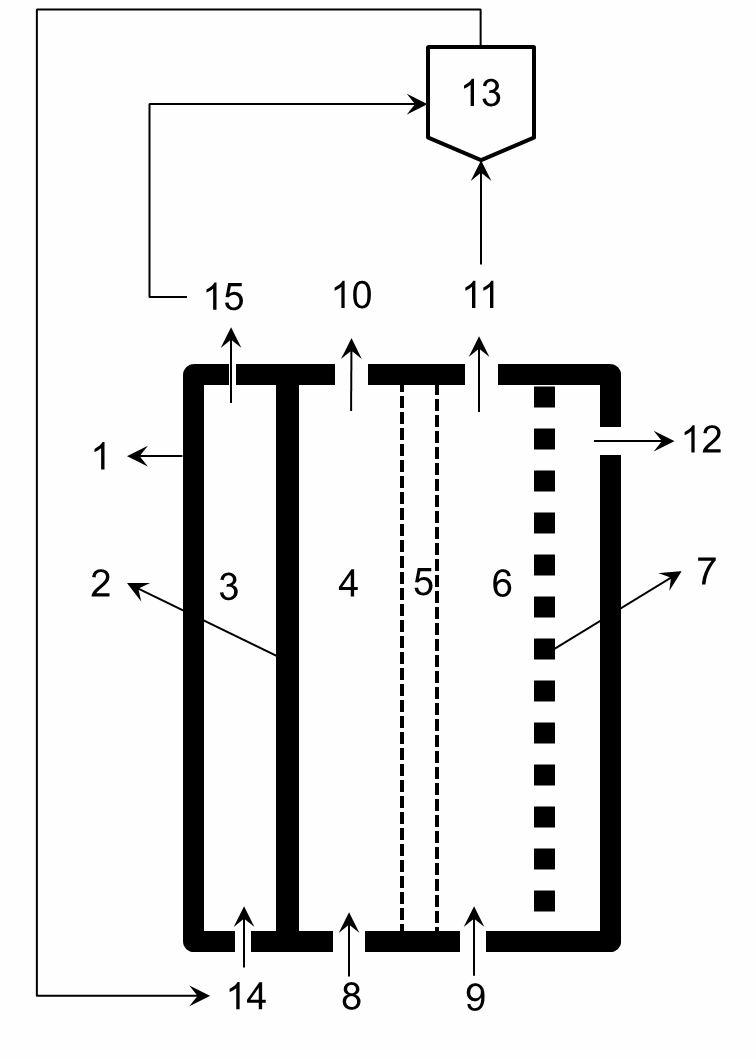
[0031] The mesh nickel ruthenium electrode is used as the cathode, and the mesh titanium ruthenium electrode is used as the anode. The effective size of the electrode is 10*10cm 2 . Asahi Kasei cation exchange membrane as the diaphragm. 1.5M reagent pure Na 2 CO 3 The solution is preheated to 70°C and passed into the anode chamber of the electrolytic cell, and the 5.5M NaOH solution is preheated to 70°C and passed into the cathode chamber. After the electrolyte fills the anode chamber and the cathode chamber, temporarily stop adding the electrolyte, and slowly increase the current density from 0 to 100mA / cm within 10 minutes 2 , and then carry out constant current electrolysis. After 50min, 1.5M Na 2 CO 3 It is continuously pumped into the anode chamber of the electrolytic cell, and 5.5M NaOH is continuously pumped into the cathod...
Embodiment 2
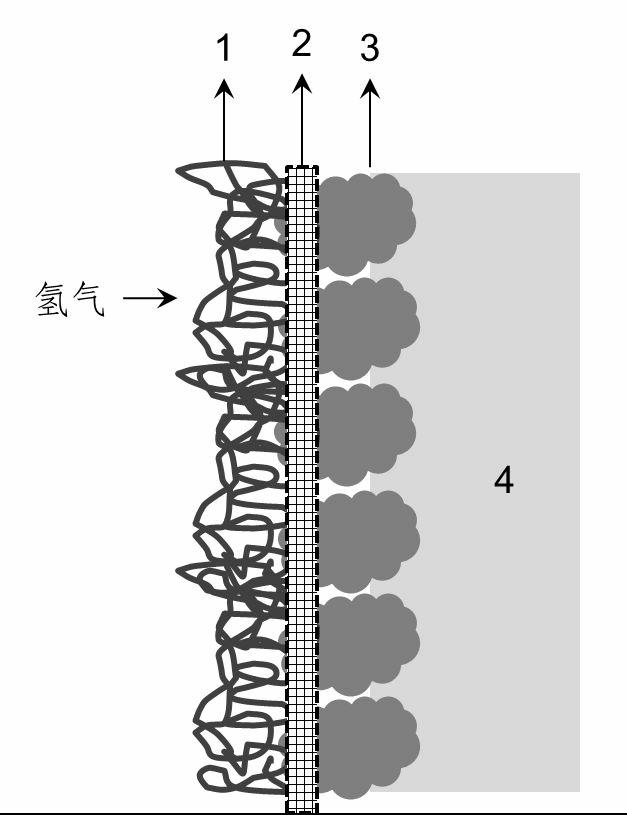
[0033] The mesh-shaped nickel-ruthenium electrode is used as the cathode, and the electrode with conductive activated carbon supported Pt-Pd as the catalyst is the hydrogen anode. The effective size of the electrode is 10*10cm 2 . Asahi Kasei cation exchange membrane as the diaphragm. 1.5M reagent pure Na 2 CO 3 The solution is preheated to 70°C and passed into the anode chamber of the electrolytic cell, and the 5.5M NaOH solution is preheated to 70°C and passed into the cathode chamber. After the electrolyte fills the anode chamber and the cathode chamber, stop adding the electrolyte temporarily, and then pass hydrogen gas with a gauge pressure of 10kPa into the hydrogen chamber, the hydrogen flow rate is about 200mL / min, and the excess unused hydrogen enters the hydrogen recovery through the upper end of the gas chamber device. The recovered hydrogen is pressurized and pumped into the cathode hydrogen chamber for use. Slowly increase the current density from 0 to 100mA / ...
Embodiment 3
[0035] The mesh-shaped nickel-ruthenium electrode is used as the cathode, and the electrode with conductive activated carbon loaded Ni-Pd as the catalyst is the hydrogen anode. The effective size of the electrode is 10*10cm 2 . Asahi Kasei cation exchange membrane as the diaphragm. After deep dealumination to remove iron and calcium containing 1.5M Na 2 CO 3 The carbon fraction mother liquor is preheated to 70°C and passed into the anode chamber of the electrolytic cell, and the 5.5M NaOH solution is passed into the cathode chamber after being preheated to 70°C. After the electrolyte is filled with the anode chamber and cathode chamber, stop adding the electrolyte temporarily, and then pass hydrogen gas with a gauge pressure of 15kPa into the hydrogen chamber, the hydrogen flow rate is about 200mL / min, and the excess unused hydrogen enters the hydrogen recovery through the upper end of the gas chamber device. The recovered hydrogen is pressurized and pumped into the cathod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com