Verapamil hydrochloride delayed-release tablet and its preparation method
A delayed-release technology for verapamil hydrochloride, which is applied in pill delivery, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of unfavorable industrial production, long production cycle, and many operation steps, and prevent serious Cardiovascular accidents, efficacy optimization, and compliance enhancement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
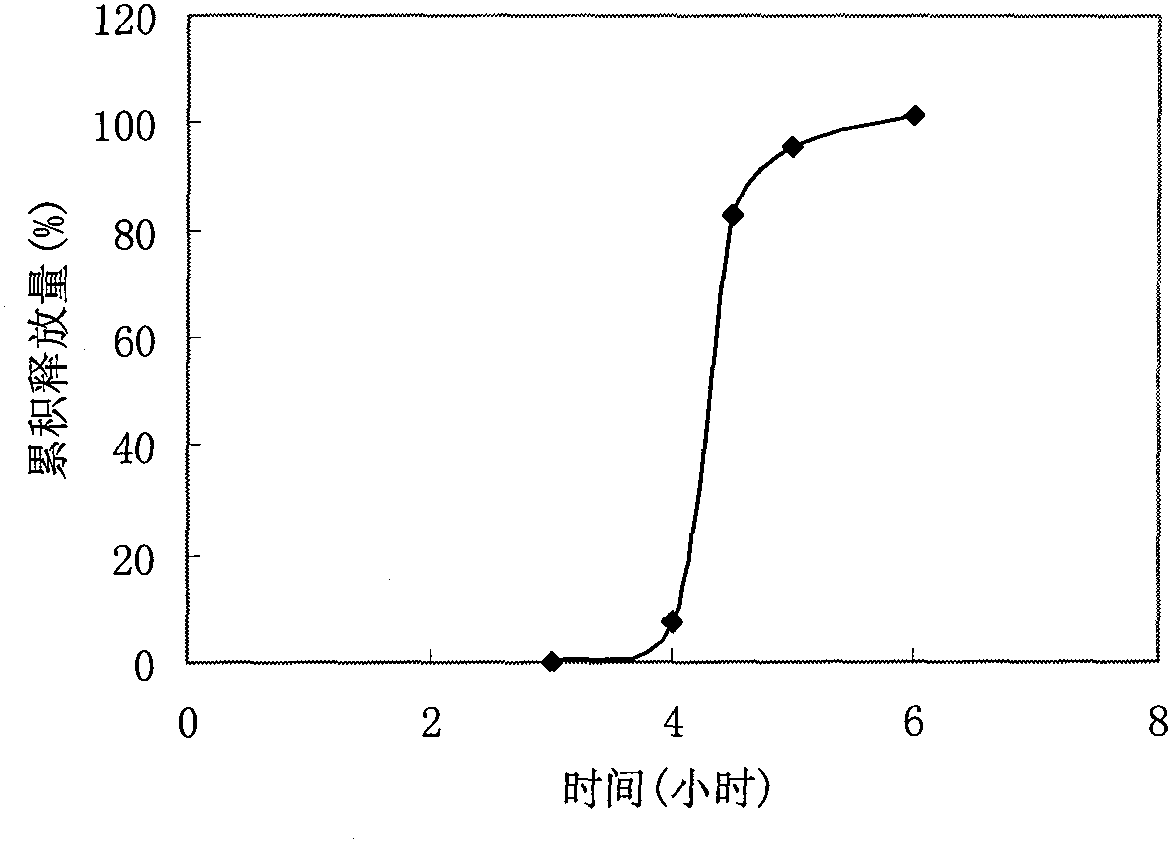
Embodiment 1
[0026] Prescription:
[0027]
[0028]
[0029] Preparation:
[0030] (1) preparation of tablet core: take prescription quantity verapamil hydrochloride and sodium carboxymethyl starch, mix, make soft material with 5% PVP ethanol solution as binding agent, cross 20 mesh sieve granulation, Dry at 40-50°C for 2 hours, pass through a 18-mesh sieve for granulation, add 1% magnesium stearate, mix well, and compress in a shallow concave die to obtain tablet cores.
[0031] (2) Preparation of coated granules: Weigh the prescription amount of HPMC E15, add 5% PVP alcohol solution as a binder to make a soft material, pass through a 40-mesh sieve for granulation, dry at 50°C for 2 hours, pass through the same mesh Sieve the whole grain, add 1% magnesium stearate, mix well, set aside.
[0032] (3) Compression coating: use a core-coating tablet machine, place half of the coated granules in the die hole of the tablet machine, then place the tablet core obtained in step (1) in the ...
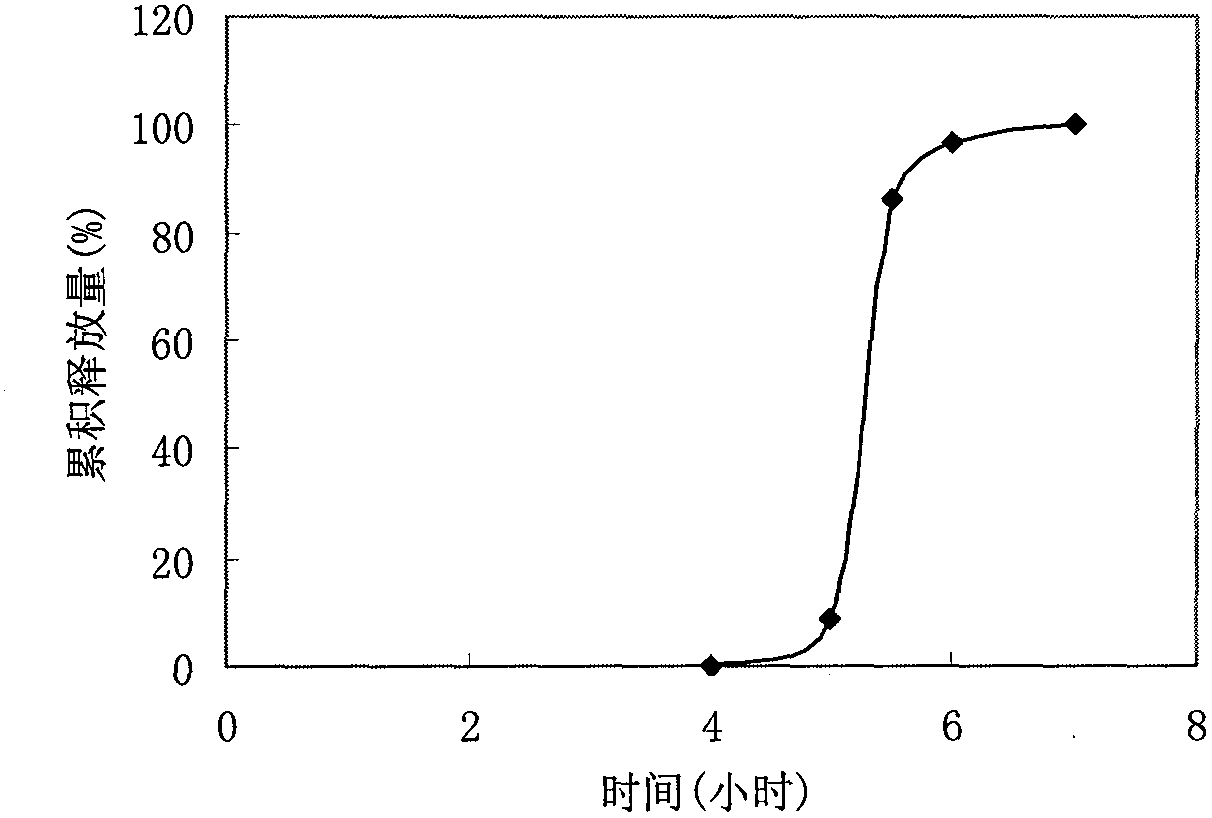
Embodiment 2
[0038] Prescription:
[0039]
[0040] Preparation:
[0041] (1) Preparation of tablet core: take by weighing auxiliary materials such as prescription amount of verapamil hydrochloride, croscarmellose sodium, mix evenly, make soft material with 10% starch slurry as binding agent, cross 20 mesh sieves Granulate, dry at 50°C for 2 hours, sieve through a 18-mesh sieve for granulation, add magnesium stearate and talc, mix well, and compress in a shallow concave die to obtain tablet cores.
[0042] (2) Preparation of coated granules: take the prescription amount of ethyl cellulose and PEG4000, add 5% HPMC alcohol solution as a binder, make soft material, pass through a 20 mesh sieve and granulate, dry at 50°C for 1 hour, Sieve through the same mesh size for granulation, add 1% sodium lauryl sulfate, mix well, and set aside.
[0043] (3) Compression coating: use a core-coating tablet machine, place half of the coated granules in the die hole of the tablet machine, then place t...
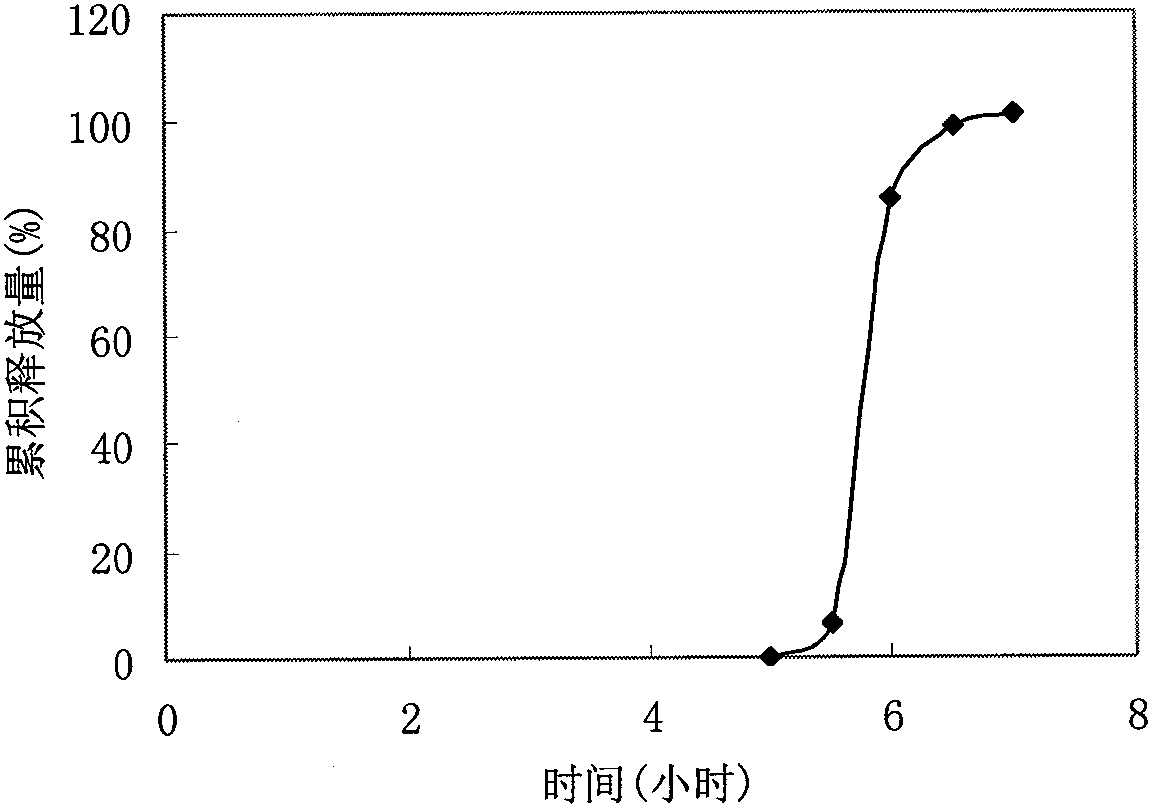
Embodiment 3
[0049] Prescription:
[0050]
[0051]
[0052] Preparation:
[0053] (1) Preparation of tablet core: take by weighing prescription quantity verapamil hydrochloride, PVPP, MCC, mix, make soft material with 50% ethanol solution as binding agent, cross 20 mesh sieves and granulate, in 40 ~ Dry at 50°C for 2 hours, sieve through a 20-mesh sieve for granulation, add magnesium stearate, mix well, and compress into tablets in a shallow concave die to obtain tablet cores.
[0054] (2) Preparation of coated granules: Take stearyl alcohol, hydrogenated castor oil, and mannitol in the prescribed amount and mix them evenly. After heating and melting, place them at room temperature to cool and solidify, grind and pulverize, pass through a 40-mesh sieve, add magnesium stearate, and Alkyl sodium sulfate mixed, set aside.
[0055] (3) Compression coating: use a core-coating tablet machine, place half of the coated granules in the die hole of the tablet machine, then place the tablet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com