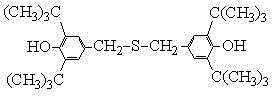Method for preparing sulfur-containing bisphenol compound antioxidant by means of recycling mother liquor
A kind of technology of sulfur-containing bisphenol, cyclic preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
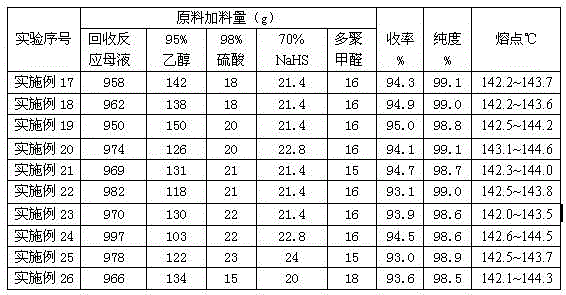
[0050] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 28.0g of paraformaldehyde, 1236g of 80% ethanol aqueous solution, 20g of sodium hydroxide, After nitrogen replacement, 25 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 50~53°C, stop heating, keep the temperature for 70 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 98.7%, melting point: 142.1~143.6℃, yield 94.5%
Embodiment 2
[0052] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 32.0g of paraformaldehyde, 1030g of 83% ethanol aqueous solution, 20g of sodium hydroxide, After nitrogen substitution, 18 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 54~56°C, stop heating, keep warm for 50 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 99.0%, melting point: 142.6~143.5℃, yield 95.2%
Embodiment 3
[0054] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 34.0g of paraformaldehyde, 825g of 85% ethanol aqueous solution, 16g of sodium hydroxide, After nitrogen substitution, 15 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 58~60°C, stop heating, keep warm for 40 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 98.5%, melting point: 142.3~143.8℃, yield 92.8%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com