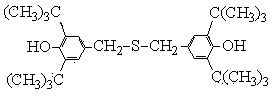Method for preparing sulfur-containing bisphenol compound antioxidant by means of recycling mother liquor
A sulfur-containing bisphenol, recycling technology, applied in the field of polyolefin plastics, lubricating oil, and synthetic rubber, can solve the problems of personnel poisoning and injury, affecting the performance of polymers, and large investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
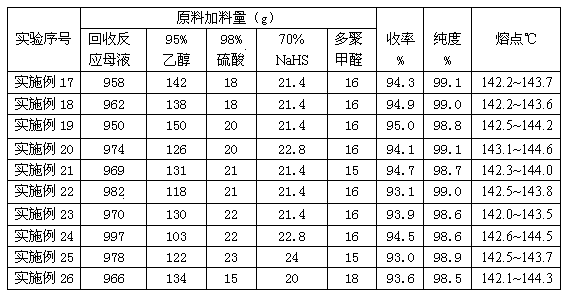
[0050] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 28.0g of paraformaldehyde, 1236g of 80% ethanol aqueous solution, 20g of sodium hydroxide, After nitrogen replacement, 25 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 50~53°C, stop heating, keep the temperature for 70 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 98.7%, melting point: 142.1~143.6℃, yield 94.5%
Embodiment 2
[0052] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 32.0g of paraformaldehyde, 1030g of 83% ethanol aqueous solution, 20g of sodium hydroxide, After nitrogen substitution, 18 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 54~56°C, stop heating, keep warm for 50 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 99.0%, melting point: 142.6~143.5℃, yield 95.2%
Embodiment 3
[0054] In the GST-2 type stainless steel autoclave with magnetic stirring, 103g (0.5mol) of raw materials 2,6-di-tert-butylphenol, 34.0g of paraformaldehyde, 825g of 85% ethanol aqueous solution, 16g of sodium hydroxide, After nitrogen substitution, 15 g of hydrogen sulfide was added. Start stirring and heating, and fully mix the reaction raw materials in the kettle. When the temperature rises to 58~60°C, stop heating, keep warm for 40 minutes, and the reaction ends. Cool down to 3~6°C, filter, separate the reaction mother liquor, and collect; the separated methylene-4426-S solid product is washed with 70~80% ethanol aqueous solution to obtain methylene-4426-S with granular pure white crystals Antioxidant product, HPLC purity: 98.5%, melting point: 142.3~143.8℃, yield 92.8%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com