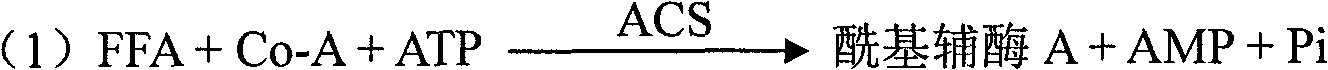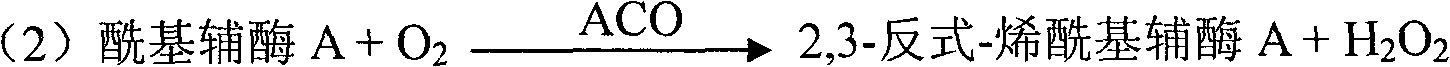Kit and method for clinically detecting serum or plasma free fatty acid
A technology of free fatty acids and kits, applied in the fields of biochemistry and testing reagents, can solve the problems of kits with narrow linear range, decreased reactivity, narrow linear range, etc., to improve stability and sensitivity, improve stability, and linearity wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of sulfhydryl reagent:
[0064]
[0065] In a clean glass container, first add the HEPES buffer (GOOD's buffer is zwitterionic buffer, including HEPES buffer, MES buffer, MOPS buffer, etc.) that has been weighed according to the formula ratio, and then add 300ml / L in sequence NEM, 600ml / L DTNB, 40ml / L EDTA, 50ml / L MIT, add appropriate amount of water, add 5ml / L Triton X-100, adjust the pH to pH 6~9 with concentrated hydrochloric acid, the dosage is about 5ml / L, and then Stir and filter, and then add distilled water to the obtained filtrate to quantify.
[0066] Preparation of Acyl-CoA Synthetase and Acyl-CoA Oxidase:
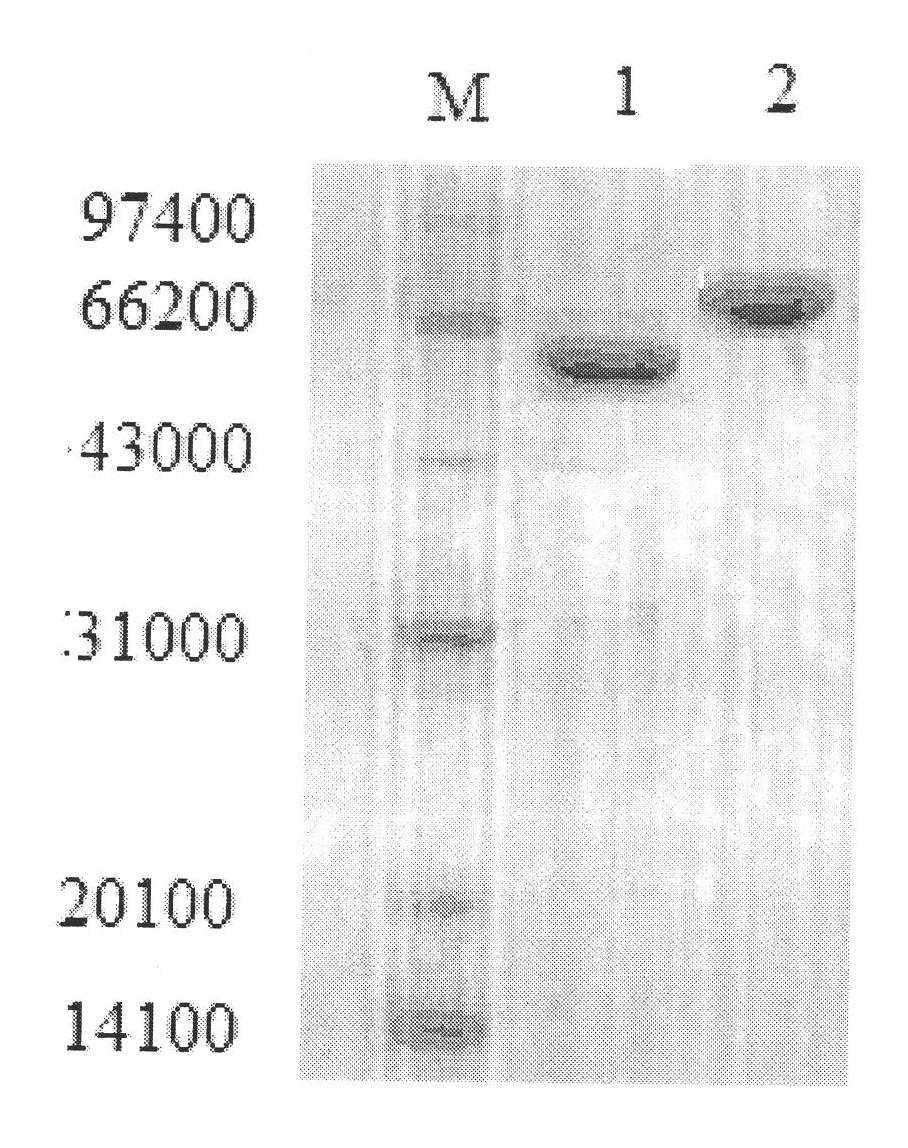
[0067] (1) Preparation of acyl-CoA synthetase: culture MK11-1 strain, collect cells, sonicate, centrifuge to get supernatant, precipitate with 30-70% ammonium sulfate, isoelectric point precipitation, DEAE-Cellulose 32 column chromatography, After molecular sieve chromatography, it was verified by SDS-PAGE that it was a single band with a...
Embodiment 2
[0076] Preparation of sulfhydryl reagent:
[0077]
[0078] Preparation method is with embodiment 1.
[0079] The preparation of acyl-CoA synthetase and acyl-CoA oxidase is the same as in Example 1.
[0080] A kit for clinical detection of free fatty acids in serum or plasma, consisting of the following two reagents:
[0081] Reagent 1:
[0082]
[0083]
[0084] Reagent 2:
[0085]
Embodiment 3
[0087] Preparation of sulfhydryl reagent:
[0088]
[0089] Preparation method is with embodiment 1.
[0090] The preparation of acyl-CoA synthetase and acyl-CoA oxidase is the same as in Example 1.
[0091] A kit for clinical detection of free fatty acids in serum or plasma, consisting of the following two reagents:
[0092]Reagent 1:
[0093]
[0094] Reagent 2:
[0095]
[0096] When measuring the sample, use the two-point endpoint method, the temperature is 37 ° C, the dominant wavelength is 550 nm, the reaction direction is upward (positive reaction), and the sample addition steps are as follows:
[0097] Sample 4μL
[0098] Reagent 1 200μL
[0099] Mix well, incubate at 37°C for 3-5min, and read the absorbance value A1
[0100] Reagent 2 50μL
[0101] Mix well, incubate for 3-5min, read absorbance A2
[0102] ΔA 样本 =A2-A1
[0103]
[0104] Using a fully automatic biochemical analyzer, the instrument will automatically give the concentration of free ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com