Preparation method of double-hydrophilic temperature response polymer
A temperature-responsive polymer technology, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, genetic material components, etc., can solve problems such as high experimental conditions, complex preparation process of stimuli-responsive polymers, and difficulties in industrialization. Achieve the effect of abundant sources, low price, and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
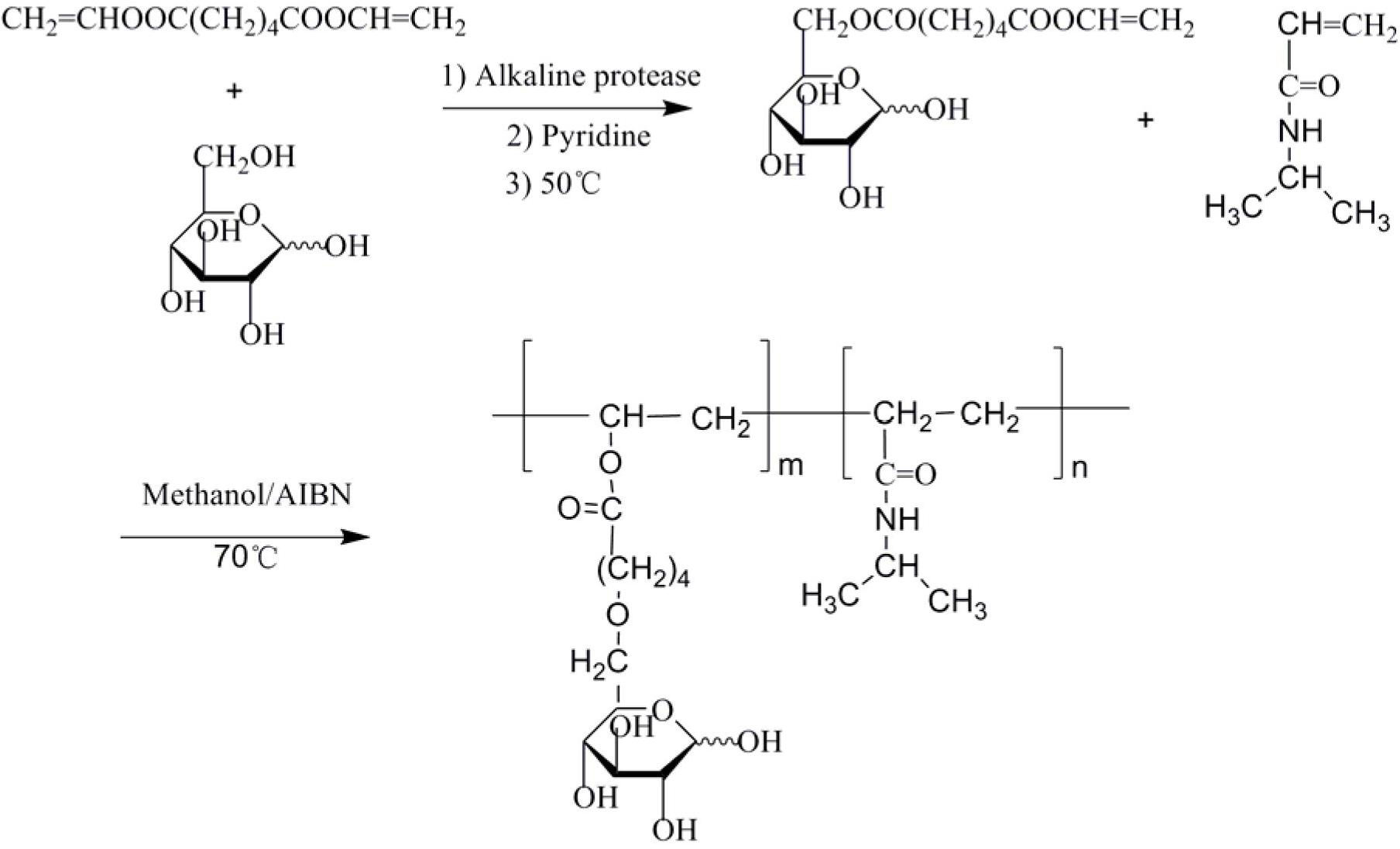
[0035] (1) Synthesis of divinyl adipate:
[0036] Mix 28g of adipic acid with 150mL of vinyl acetate, add 0.4g of copper sulfate catalyst, and react at 70°C to obtain 30g of divinyl adipate, with a yield of 78%;
[0037] (2) Enzymatic synthesis of polymerizable glucose divinyl adipate:
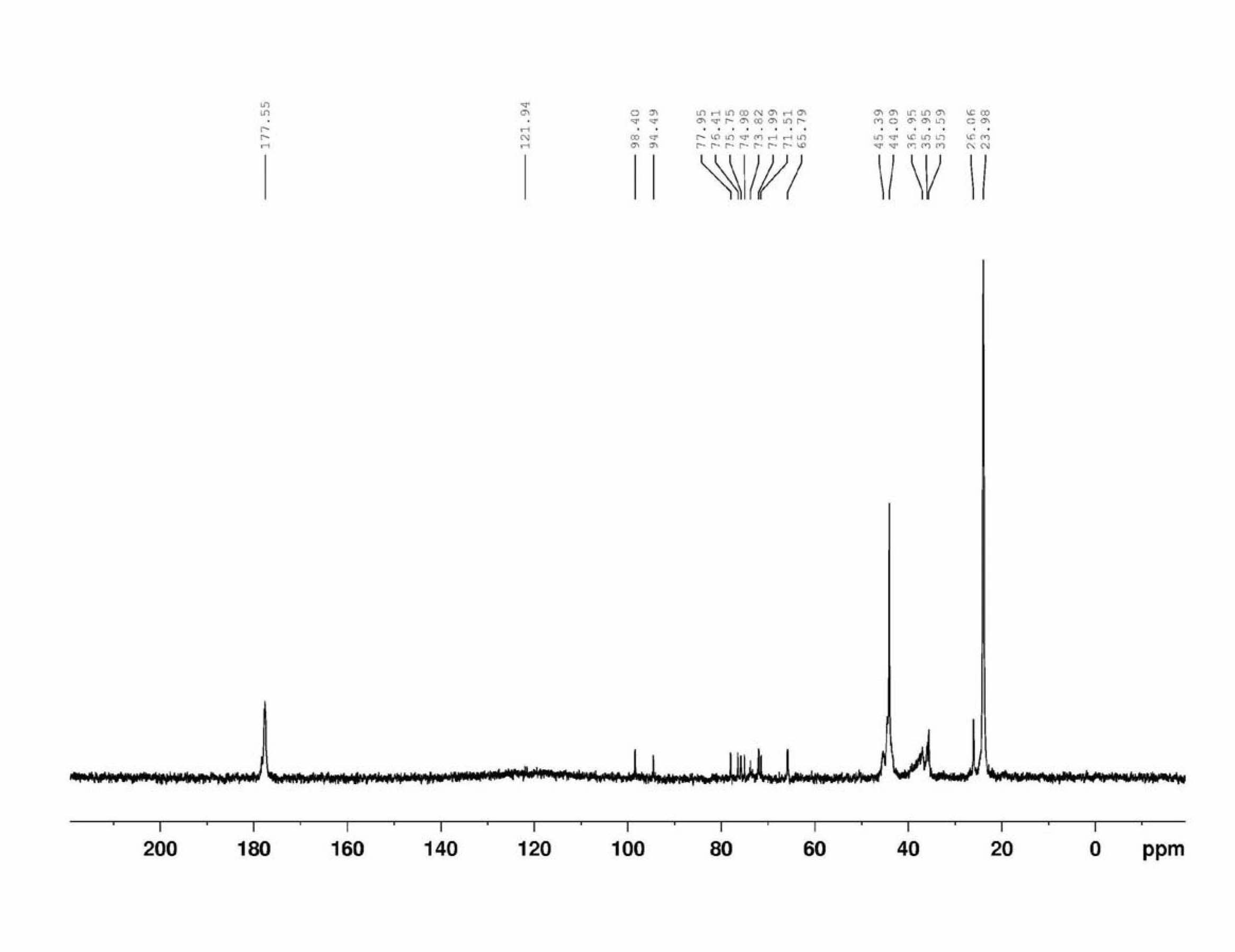
[0038] Add 13.14g of divinyl adipate (0.066mol), 3.96g of glucose, 1.0g of subtilisin and 100mL of anhydrous pyridine into a 250mL Erlenmeyer flask, and put it in a constant temperature shaking incubator at 50°C for 4 days at a speed of 210rpm . The reaction process was qualitatively monitored by thin-layer chromatography (TLC), and the developing solvent was ethyl acetate / methanol / water (17:3:1 by volume), and I 2 color. After the reaction was finished, the subtilisin was removed by filtration and most of the pyridine was removed by distillation under reduced pressure, and the crude product was purified by column chromatography, the eluent was pure ethyl acetate, and the developer was ethy...
Embodiment 2
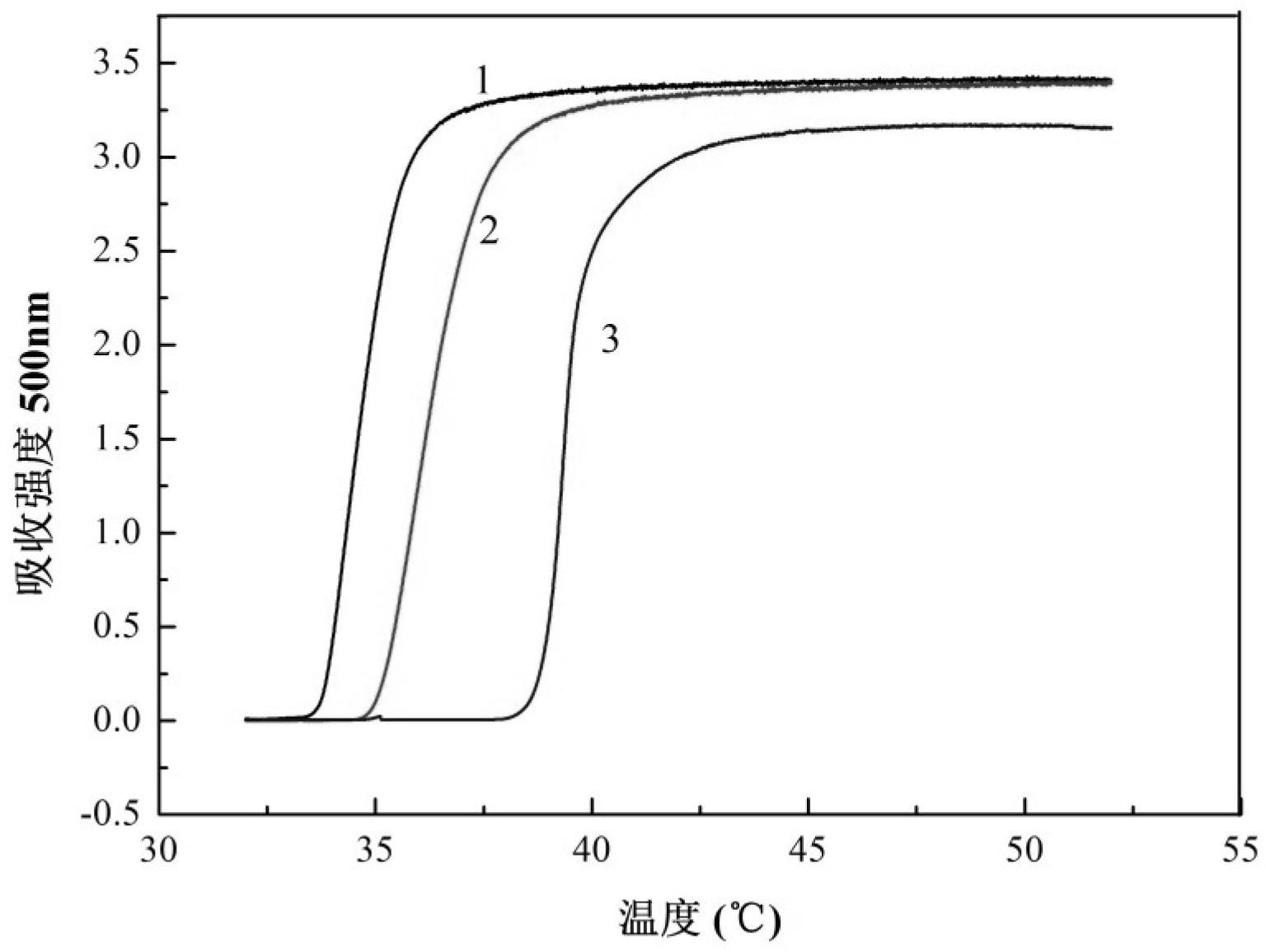
[0040] Add 3.96g of N-isopropylacrylamide, 2.34g of glucose divinyl adipate and 0.126g of AIBN into a flask equipped with a magnetic stirring rotor and an adapter, completely dissolve in 5mL of DMF, seal it, and repeatedly evacuate it. After several times of nitrogen gas, the reaction system was placed at 60°C and stirred for 24 hours under the protection of nitrogen gas, and then the viscous colorless and transparent polymer solution obtained from the reaction was precipitated in a large amount of ether, and the ether was poured out, and the white solid precipitate was again Dissolve in a small amount of ethanol, repeatedly precipitate and dissolve until all unreacted monomers are washed out, and dry in a vacuum oven at 37°C for 24 hours to obtain a white solid powder.
Embodiment 3
[0042] Add 7.92g of N-isopropylacrylamide, 2.34g of glucose divinyl adipate and 0.206g of AIBN into a flask equipped with a magnetic stirring rotor and an adapter, dissolve it completely in 10mL of DMF, seal it, and repeatedly evacuate it. After several times of nitrogen gas, the reaction system was placed at 60°C and stirred for 24 hours under the protection of nitrogen gas, and then the viscous colorless and transparent polymer solution obtained from the reaction was precipitated in a large amount of ether, and the ether was poured out, and the white solid precipitate was again Dissolve in a small amount of ethanol, repeatedly precipitate and dissolve until all unreacted monomers are washed out, and dry in a vacuum oven at 37°C for 24 hours to obtain a white solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com