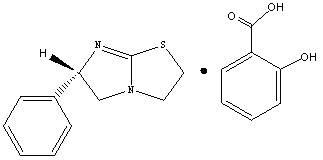
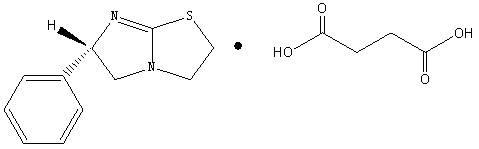
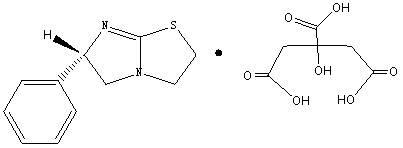
Levamisole organic acid salt, synthetic method for levamisole organic acid salt and medicinal composition of levamisole organic acid salt
A technology of levamisole and organic acid salts, which is applied in the field of medicine, can solve the problems of reducing the stability of finished products, low bioavailability, and adding related substances, and achieve the effects of improving thermal stability, high bioavailability, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Add 48 g of levamisole hydrochloride (II) and 200 ml of distilled water into the reaction flask, stir to dissolve, add 200 ml of 1 mol / L NaOH aqueous solution dropwise in an ice bath, stir until the solution pH=7~12, and heat in a water bath to 40°C and continue stirring for 30 minutes. Filter, wash the filter cake with distilled water until neutral, and dry under reduced pressure to obtain levamisole free base (Ⅲ). The filtrate was extracted three times with dichloromethane, 160ml for the first time, and 120ml for the second and third times. The extracts were combined, and distilled water was added to the extracts, stirred and cleaned, left to separate, and washed repeatedly until the lotion was weakly alkaline. Dry with anhydrous calcium chloride for 6h and filter. Dichloromethane was distilled off under reduced pressure to obtain levamisole free base (Ⅲ) as a white solid, which was combined with levamisole free base.
[0073] In the dry reaction bottle, ...
Embodiment 2
[0074] Example 2: Add 48g of levamisole hydrochloride (II) and 200ml of distilled water into the reaction bottle, stir to dissolve, add dropwise 360ml of 1mol / L NaOH aqueous solution under the condition of ice bath, stir until the solution pH=7~12, and heat in a water bath To 40°C, continue stirring for 5.5h. Filter, wash the filter cake with distilled water until neutral, and dry under reduced pressure to obtain levamisole free base (Ⅲ). The filtrate was extracted three times with ether, 240ml for the first time, 90ml for the second and third times, combined the extracts, then added distilled water to the extracts, stirred and cleaned, left to separate, and repeated cleaning until the lotion was weakly alkaline. Calcium chloride water dried for 6h, filtered. Diethyl ether was distilled off under reduced pressure to obtain levamisole free base (Ⅲ) as a white solid, which was combined with levamisole free base.
[0075] In the dry reaction bottle, add 30ml of anhydrous methan...
Embodiment 3
[0076] Example 3: Add 48g of levamisole hydrochloride (II) and 200ml of distilled water into the reaction flask, stir to dissolve, add dropwise 400ml of 1mol / L NaOH aqueous solution under the condition of ice bath, stir until the solution pH=7~12, and heat in a water bath To 25°C, continue stirring for 3h. Filter, wash the filter cake with distilled water until neutral, and dry under reduced pressure to obtain levamisole free base (Ⅲ). The filtrate was extracted three times with a mixed solvent of dichloromethane and diethyl ether (1:1), 320ml for the first time, 160ml for the second and third times respectively, combined the extracts, then added distilled water to the extracts, stirred and cleaned, stood to separate, and repeated Wash until the lotion is weakly alkaline, dry with anhydrous calcium chloride for 12 hours, and filter. The solvent was evaporated under reduced pressure to obtain levamisole free base (Ⅲ) as a white solid, and the levamisole free base was combined....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com