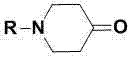
Synthesis method for N-substituted-4-piperidone
A synthesis method and piperidone technology are applied in the field of synthesis of N-substituent-4-piperidone compounds, and can solve the problems of expensive 4-piperidone raw materials, high risk of metallic sodium, poor atom economy and the like, To achieve the effect of reducing production cost, good atomic economy and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 73 grams (1.01 mol) of acrylic acid and 7.2 grams (0.1 mol) of N,N-dimethylformamide into the reaction flask, heat up to 70°C, and add 145 grams (1.05 mol) of ) phosphorus trichloride, the dropwise addition is completed within 5 hours, and the reaction is continued for half an hour, and the reaction ends. Distilled under reduced pressure, collected fractions at 60°C / 6kPa to obtain 130 g of 3-chloropropionyl chloride (content 90.2%, yield 83.6%);
[0023] (2) Dissolve 277 g (2.08 mol) of aluminum trichloride in 250 mL of dichloroethane, cool down to 0°C, add 200 g (1.57 mol) of 3-chloropropionyl chloride dropwise for 30 minutes under constant temperature stirring, and keep When the temperature is below 5°C, ethylene gas is passed through, and after 3 hours of ventilation, stir for a while, and the reaction ends. Configure 10% hydrochloric acid aqueous solution, cool to 0°C, slowly pour the reaction solution under stirring, and keep the temperature below 10°C. S...
Embodiment 2
[0026] (1) Add 73 grams (1.01 mol) of acrylic acid and 7.2 grams (0.1 mol) of N,N-dimethylformamide into the reaction flask, heat up to 70°C, and add 125 grams (1.05 mol) of ) Thionyl chloride, the dropwise addition is completed within 5 hours, and the reaction is continued for half an hour, and the reaction ends. Distilled under reduced pressure, collected fractions at 60°C / 6kPa to obtain 130 g of 3-chloropropionyl chloride (content 90.2%, yield 83.6%);
[0027] (2) Dissolve 231 grams (1.73 mol) of aluminum trichloride in 250 mL of chloroform, cool down to 0°C, and add 200 grams (1.57 mol) of 3-chloropropionyl chloride dropwise for 30 minutes under constant temperature stirring, keeping the temperature Feed ethylene gas below 5°C for 3 hours and then stir for a while, the reaction is over. Configure 10% hydrochloric acid aqueous solution, cool to 0°C, slowly pour the reaction solution under stirring, and keep the temperature below 10°C. Static separation, the organic phase ...
Embodiment 3
[0030] (1) Add 73 grams (1.01 mol) of acrylic acid and 7.2 grams (0.1 mol) of N,N-dimethylformamide into the reaction flask, heat up to 70°C, and add 68 grams (0.53 mol) of ) Oxalyl chloride, the dropwise addition is completed within 5 hours, and the reaction is continued for half an hour, and the reaction ends. Distilled under reduced pressure, collected fractions at 60°C / 6kPa to obtain 130 g of 3-chloropropionyl chloride (content 90.2%, yield 83.6%).
[0031] (2) Dissolve 314 grams (2.36 mol) of aluminum trichloride in 250 mL of dichloromethane, cool down to 0°C, and add 200 grams (1.57 mol) of 3-chloropropionyl chloride dropwise for 30 minutes under constant temperature stirring, keeping the temperature Feed ethylene gas below 5°C for 3 hours and then stir for a while, the reaction is over. Configure 10% hydrochloric acid aqueous solution, cool to 0°C, slowly pour the reaction solution under stirring, and keep the temperature below 10°C. Static separation, the organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com