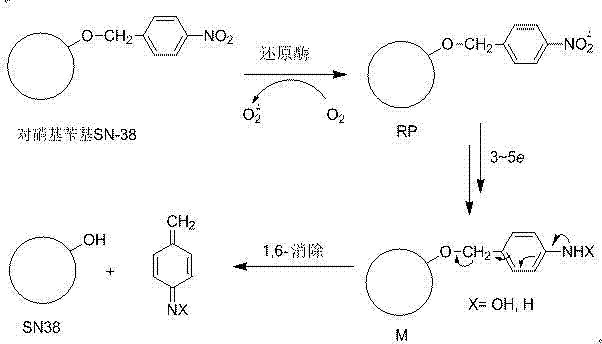
P-nitro aryl methoxycamptothecine anoxic activated prodrug used for antitumor drug
A technology of nitroarylmethoxycamptree and hypoxia-activated prodrugs, which is applied in the field of antineoplastic drugs, can solve the problems of unstable plasma metabolism, toxic and side effects, insoluble, etc., and achieve improved water solubility and stability, low Toxic and side effects, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
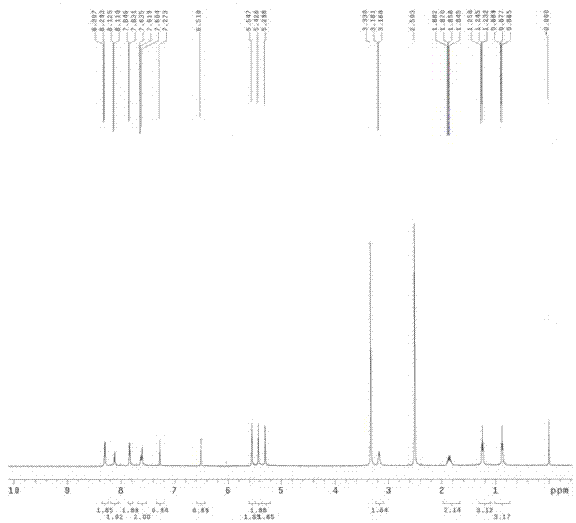
[0063] Reality Embodiment 1. p-nitrobenzyl SN-38 and preparation method thereof
[0064] 1) Chemical name of p-nitrobenzyl SN-38:
[0065] (4S)-4,11-diethyl-4-hydroxy-9-(4-nitrobenzyloxy)-1H-pyrano[3',4':6,7]indoleazino[1 ,2-b] Quinoline-3,14(4H,12H)-dione.
[0066] The chemical structural formula of p-nitrobenzyl SN-38:
[0067]
[0068] 2) The preferred preparation method of p-nitrobenzyl SN-38:
[0069] Dissolve 4.32 g of p-nitrobenzyl bromide and 3.92 g of SN-38 in 10 ml of 1,4-dioxane, add 3.28 g of cesium carbonate at room temperature, raise the temperature to 85°C after the addition, and stir for 5 hours. The system was cooled to room temperature. 200 ml of dichloromethane and 200 ml of water were added, the organic phase was separated, and the aqueous layer was extracted with dichloromethane (200 ml x 3). The organic phases were combined and dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product which...
Embodiment 2
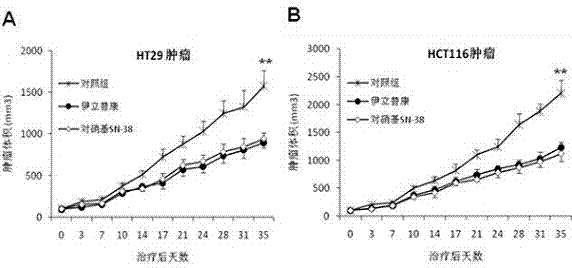
[0074] Example 2. The application effect of nitrobenzyl SN-38 and the comparison with camptothecin derivatives standard drug irinotecan
[0075] 1) Identification of anticancer activity of nitrobenzyl SN-38 and comparative analysis with irinotecan:
[0076] image 3 Shown are the growth curves of subcutaneous colon cancer tumors HCT116 and HT29 in nude mice after treatment with p-nitrobenzyl SN-38 and irinotecan, and the comparative analysis of tumor growth with the control group.
[0077] 5 × 106 human colon cancer HCT116 and HT29 cells in logarithmic growth phase were subcutaneously injected into the left flank of 6-week-old female Balb / c nude mice. When the tumor grew to 100 mm3 (day 0), the animals were randomly divided into three groups, namely the control group, the irinotecan group and the p-nitrobenzyl SN-38 group, and were given intraperitoneal injection of normal saline, irinotecan (50mg / kg, sorbitol / lactic acid buffer [45 mg / ml sorbitol / 0.9 mg / ml lactic acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com