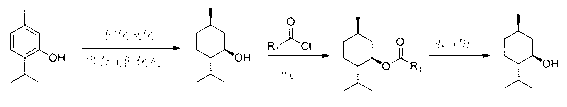Preparation method of L-menthol
A technology of menthol and menthol ester, applied in the field of preparation of bulk spice L-menthol, can solve the problems of low splitting efficiency of L-menthol, complicated purifying enzyme process, difficult to obtain raw materials, etc. Synthesis efficiency, easy operation, and the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] D, the preparation of L-menthol, concrete steps are as follows:
[0034] In a 1L autoclave with a stirrer, add thymol (250g, 1.67mol), dissolve it with 200mL of methanol, then add 2.5g of 10% palladium carbon catalyst, seal the reaction kettle, replace the air in the kettle with hydrogen , and then fill the reaction system with hydrogen to make the pressure of the reaction system to 3.0-4.0MPa, and then heat the reaction system until the reaction temperature is 120-130°C. During the reaction, the pressure of the reaction system will continue to decrease as the reaction progresses, so , continuously add hydrogen under stirring, maintain the pressure of the reaction system at 3.0-4.0 MPa, stop heating when the hydrogen is no longer absorbed, adjust the temperature of the reaction system to room temperature, stop stirring, vent the reaction kettle, open the autoclave, Take out the material, filter, and reclaim the palladium-carbon catalyst for the next reaction. The filtra...
Embodiment 2
[0036] D, the preparation of L-menthol, concrete steps are as follows:
[0037] In a 1L autoclave with a stirrer, add thymol (250g, 1.67mol), dissolve it with 200mL of ethanol, then add 5gW-2 Raney nickel catalyst, seal the reactor, and replace the air in the reactor with hydrogen , and then fill the reaction system with hydrogen to make the pressure of the reaction system to 3.0-4.0MPa, and then heat the reaction system until the reaction temperature is 120-130°C. During the reaction, the pressure of the reaction system will continue to decrease as the reaction progresses, so , continuously add hydrogen under stirring, maintain the pressure of the reaction system at 3.0-4.0 MPa, stop heating when the hydrogen is no longer absorbed, adjust the temperature of the reaction system to room temperature, stop stirring, vent the reaction kettle, open the autoclave, Take out the material, filter, reclaim the Raney nickel catalyst and can be used for the next reaction, the filtrate is co...
Embodiment 3
[0039] D, the preparation of L-menthol, concrete steps are as follows:
[0040] In a 1L autoclave with a stirrer, add thymol (250g, 1.67mol), dissolve it with 200mL of ethanol, add 5g of copper-chromium catalyst, seal the reactor, replace the air in the reactor with hydrogen, and then fill Hydrogen makes the pressure of the reaction system to 3.0-4.0MPa, and then heats the reaction system until the reaction temperature is 120-130°C. During the reaction, as the reaction progresses, the pressure of the reaction system will continue to decrease. Therefore, when stirring Continue to add hydrogen under high pressure to maintain the pressure of the reaction system at 3.0-4.0MPa. When the hydrogen is no longer absorbed, stop heating, adjust the temperature of the reaction system to room temperature, stop stirring, vent the reactor, open the autoclave, and take out the materials. Filtration, recovery of copper chromium catalyst can be used for the next reaction, the filtrate is concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com