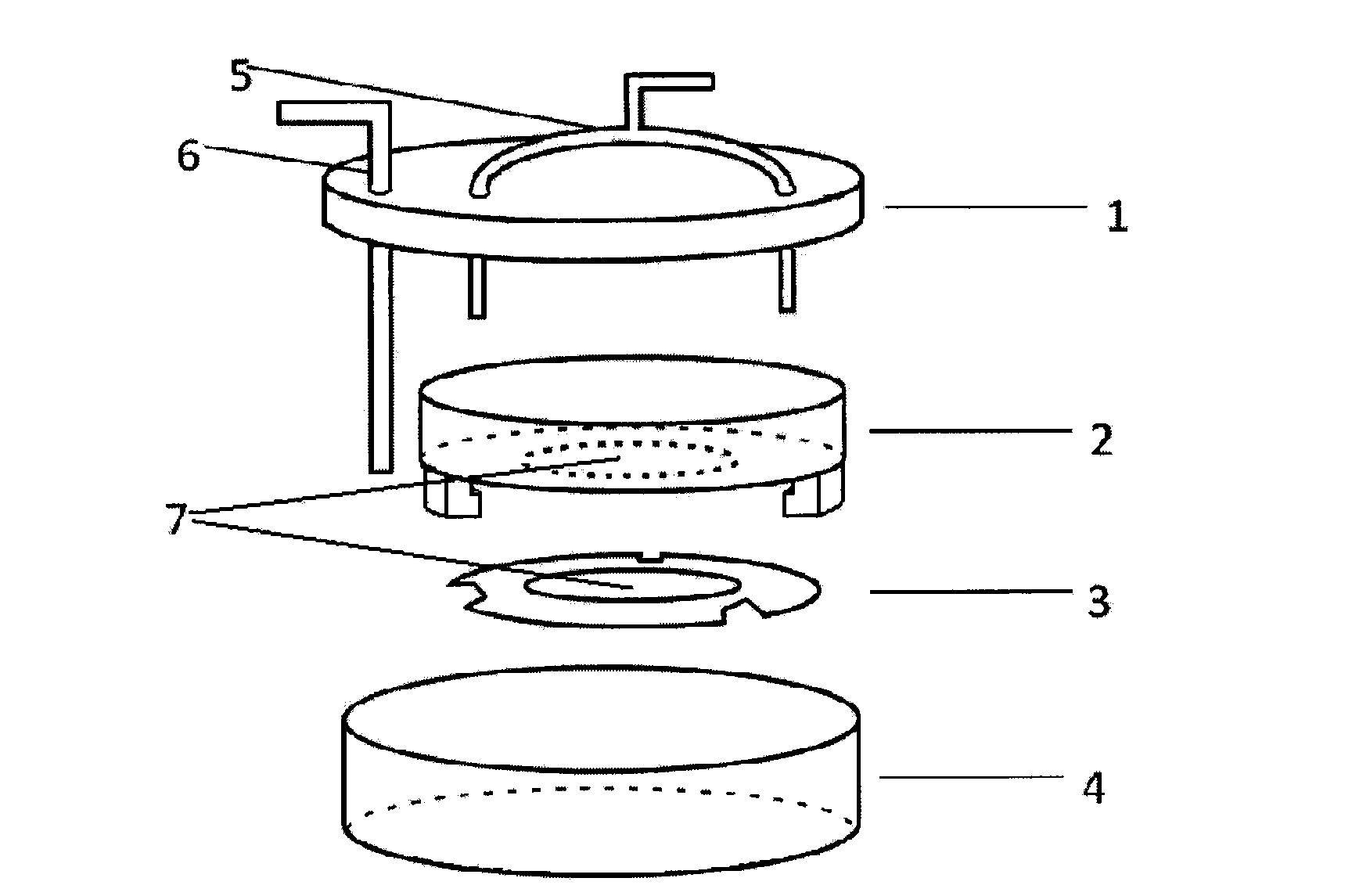
Method for preparing tissue engineered cornea and device of method
A technology of tissue engineering and cornea, which is applied in the field of tissue engineering cornea preparation for clinical transplantation, can solve the problems of large corneal epithelial defect, increased rejection risk, immune rejection, etc. The effect of layers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step 1. Inoculation of amnion epithelial cells: place the decellularized porcine corneal stroma in a 12-well cell culture plate, and inoculate the second-generation amnion epithelial cells cultured in culture solution A at 1×10 5 / cm 2 Density seeding on the Bowman's membrane surface of the decellularized corneal stroma; add 2ml of culture medium A, and cultivate under the liquid surface; the culture medium A is based on the MCDB153 medium aqueous solution with a concentration of 17.6g / L, and contains: EGF 2μg / L, human insulin 10mg / L, L-glutamine 2mM, non-essential amino acid 10ml / L, sodium pyruvate 1.18g / L, arachidonic acid 2mg / L, adenine 10mg / L, triiodine Thyrosine 20ng / L, sodium selenite 5mg / L, transferrin 100mg / L; the condition of cell incubator is, in the volume concentration respectively is 90% N 2 , 5% CO 2 , 5% O 2 In a mixed gas environment, culture at 37°C for 7 days; replace the culture medium every two days, and when the amniotic epithelial cells grow and...
Embodiment 2
[0041] Step 1. Inoculation of amnion epithelial cells: place decellularized bovine corneal stroma in a 12-well cell culture plate, press 8×10 5 / cm 2 Density inoculation of primary amniotic epithelial cells on the surface of the Bowman's membrane of the decellularized corneal stroma; Add 4ml of culture solution A and culture under the liquid surface; the culture solution A is the same as in Example 1; the conditions of the cell culture box are, in volume Concentrations were 93% N 2 , 5% CO 2 , 2% O 2 In a mixed gas environment, culture at 37°C for 5 days; change the medium every two days, and when the amniotic epithelial cells grow and confluence on the surface of the descemet layer of the decellularized corneal stroma, the cells grow to 1-2 layers. A construct in which amniotic epithelial cells were complexed on the surface of the decellularized corneal stroma was obtained.
[0042] Step 2, air-liquid alternate culture of epithelial cell membrane: same as Example 1, use t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com