pH fluorescent sensor based on allyl calcein and preparation method thereof
A fluorescent sensor, allyl calcium technology, applied in the field of fluorescent sensors to achieve excellent selectivity, good stability, and prevent leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the synthesis of allyl calcein
[0021] Allyl Calcein Synthetic Step Reference: The synthetic route synthesis in the Journal of Wuhan Institute of Chemical Technology, 1998:V20 15-17, the specific steps are as follows:
[0022] (1) Weigh 500mg of calcein, dissolve it in NaOH solution with pH=13, add calcium chloride at a ratio of 1:1.1 (molar ratio), after it is completely dissolved, adjust the pH with NaOH to make the pH value greater than 12. Add 0.4ml bromopropene and add 0.1ml 18-crown-6 ether at the same time, and react on DF-101B collector type constant temperature magnetic stirrer at 60°C for 3 hours.
[0023] (2) Adjust the reacted solution to acidity with HCl. After the solution turns yellow, put it in the refrigerator to cool. The desired product (MS: 657) can be obtained by vacuum drying at 70°C for 6 hours after suction filtration the next day.
[0024] Under alkaline conditions, the hydrogen ions on the 5 carboxyl groups of allyl calcein ar...
Embodiment 2
[0026] The preparation and determination of the fluorescent sensor include the following steps:
[0027] 1. Silanization of slides: slides (diameter 12.5 mm) were soaked in chromic acid lotion for 40 minutes, then soaked in 3% hydrofluoric acid for 30 minutes, and then rinsed with distilled water so that they were no longer stained with 3 % hydrofluoric acid, then add 10% hydrogen peroxide and soak for 30 minutes. Then rinse with distilled water. Measure 0.2ml propyl methacrylate with a dry pipette, 2ml0.2mol l -1 Mix acetic acid-sodium acetate buffer solution with a pH of 3.6 and 8 ml of distilled water, and stir for 10 minutes to completely dissolve the propyl methacrylate to prepare a silylation solution. (Except for the beaker used for immersion in the chromic acid lotion, the other containers used are all plastic containers) The slides are immersed in this solution for 2-3 hours, then washed with distilled water, and dried at room temperature for later use.
[0028] 2....
Embodiment 3
[0031] Embodiment 3: Determination of correction equation
[0032] Solutions with different pH values are B-R buffer solutions, prepared with phosphoric acid, acetic acid, boric acid, and 0.2mol l -1 NaOH solution to adjust the pH to the desired value.
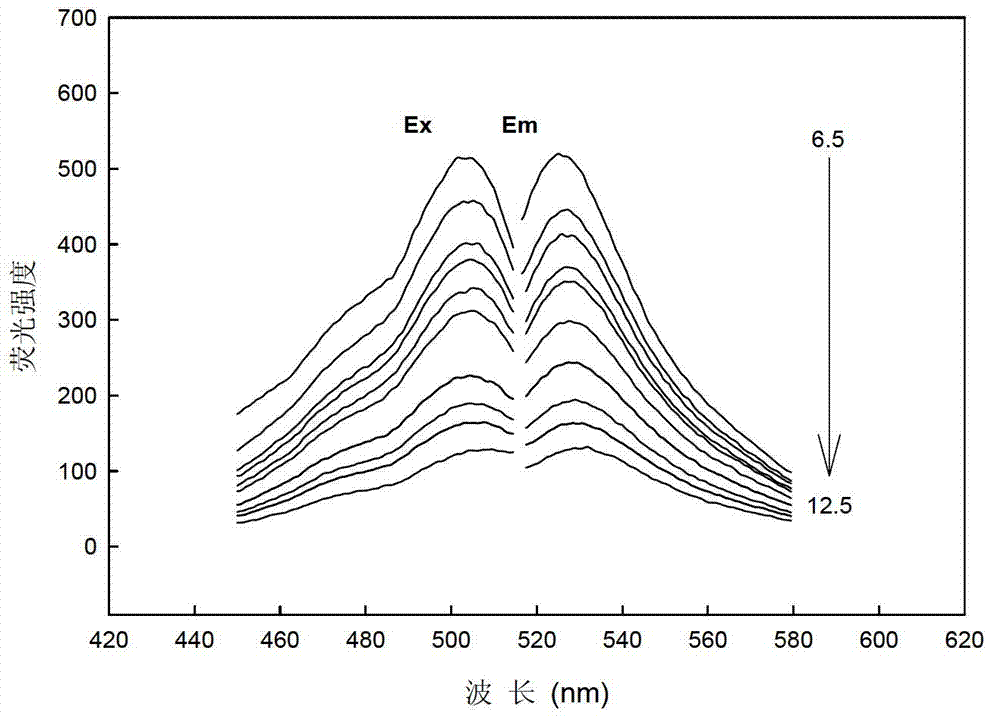
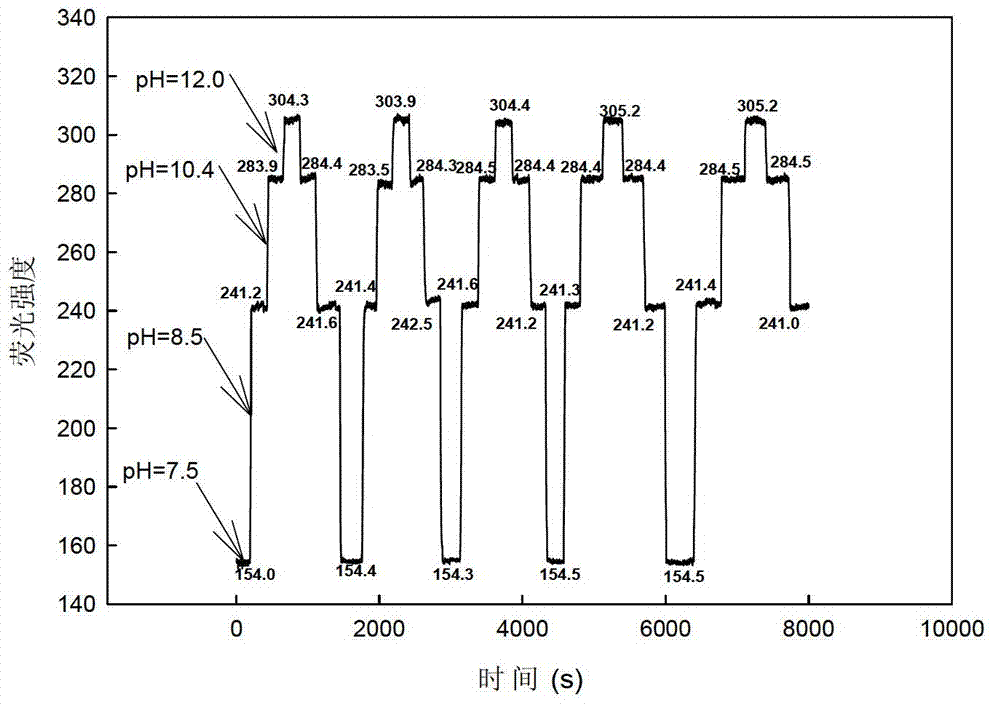
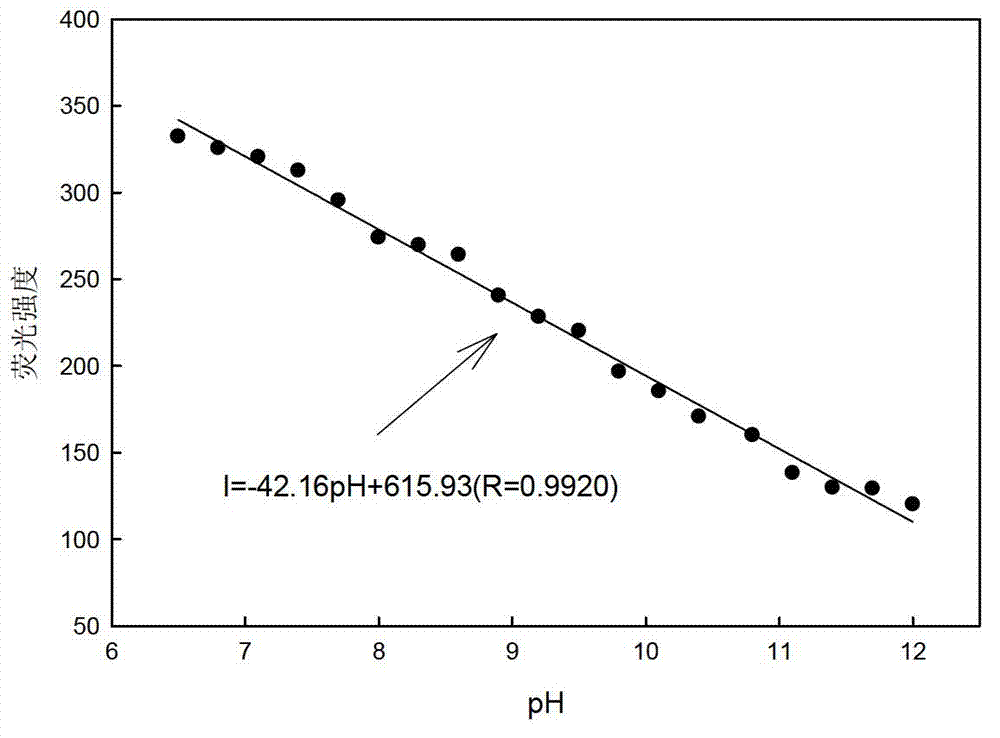
[0033] The photoelectrode film made of allyl calcein as the fluorescent carrier was put into the polytetrafluoroethylene flow cell, and the B-R buffer solutions with different pH values (pH=6.5, 6.8, 7.1, 7.4, 7.7, 8.0, 8.3, 8.6, 8.9, 9.2, 9.5, 9.8, 10.1, 10.4, 10.8, 11.1, 11.4, 11.7, 12.0), the peristaltic pump is input into the flow cell at a speed of 1.5ml / min, at the maximum excitation wavelength of 505nm and the maximum emission wavelength of 526nm Measure the fluorescence intensity (I) of the solution, and use Sigmaplot software to make a straight line fitting, such as image 3 As shown, the correction equation obtained is: I=-42.16pH+615.93 (R=0.9920). Therefore, we can measure the value of fluorescence intensity a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com