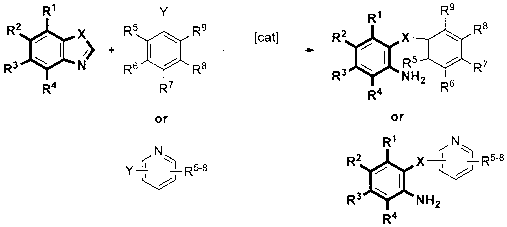
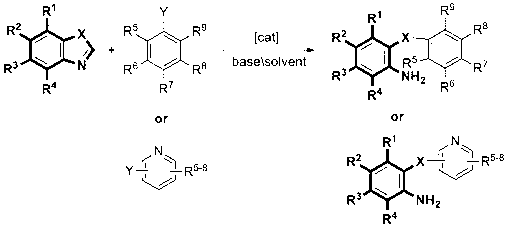
Method for synthesizing o-amino diaryl ether and o-amino diaryl sulfur ether
A technology of o-amino diaryl sulfide and o-amino diaryl ether, which is applied in chemical instruments and methods, formation/introduction of amino groups, preparation of amino hydroxy compounds, etc. problem, to achieve the effect of direct reaction, simple method and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
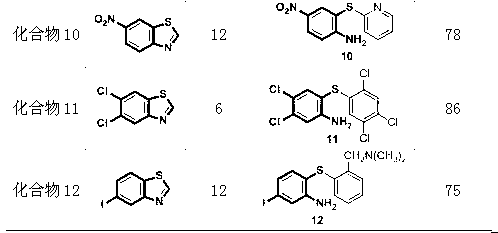
[0039] Compound 1: Add metal copper powder (0.05mmol), cesium carbonate (1.0mmol) and polyethylene glycol-600 (2.0g) into a 25mL reaction flask, fill with nitrogen protection, add benzothiazole (0.5mmol) and iodobenzene (1.0 mmol). The reaction mixture was at 140 o Reaction at C until the reaction of the raw materials is complete. After cooling to room temperature, the solvent was evaporated under reduced pressure and separated by column chromatography (petroleum ether: diethyl ether: triethylamine = 30: 1: 1) to obtain a pale yellow liquid. Yield 96%.
Embodiment 2
[0041] Compound 2: Add metal copper powder (0.05mmol), cesium carbonate (1.0mmol) and polyethylene glycol-600 (2.0g) into a 25mL reaction flask, fill with nitrogen protection, add benzothiazole (0.5mmol) and o-iodine Toluene (1.0 mmol). The reaction mixture was at 140 o Reaction at C until the reaction of the raw materials is complete. After cooling to room temperature, the solvent was evaporated under reduced pressure and separated by column chromatography (petroleum ether: diethyl ether: triethylamine = 30: 1: 1) to obtain a pale yellow liquid. Yield 94%.
Embodiment 3
[0043] Compound 3: Add metal copper powder (0.05mmol), sodium carbonate (1.0mmol) and polyethylene glycol-400 (2.0g) to a 25mL reaction flask, fill with nitrogen protection, add benzothiazole (0.5mmol) and p-iodine Aniline (1.0 mmol). The reaction mixture was at 140 o Reaction at C until the reaction of the raw materials is complete. After cooling to room temperature, the solvent was evaporated under reduced pressure and separated by column chromatography (petroleum ether: diethyl ether: triethylamine = 30: 1: 1) to obtain a pale yellow liquid. Yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com