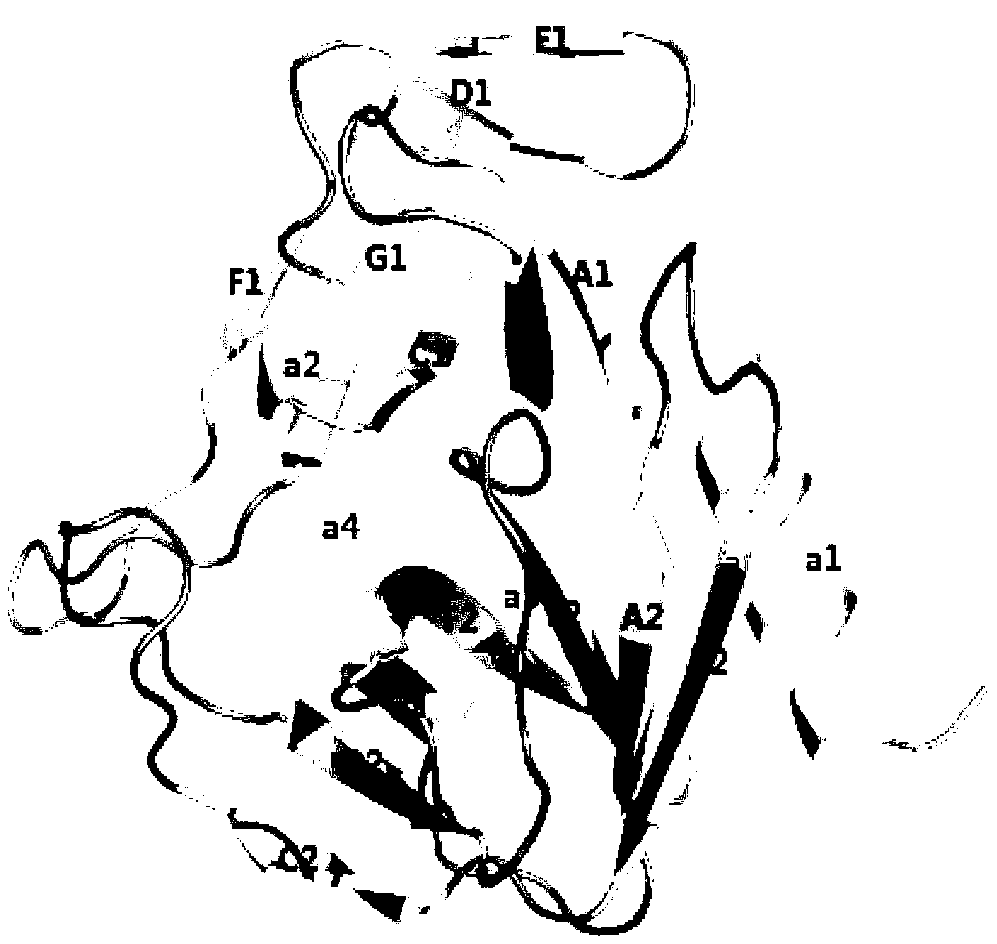Crystal structure of enterovirus 71 3C protease and application thereof
A technology of crystal structure and protease, applied in the field of protease, can solve the problem of lack of inhibitor molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Expression and purification of enterovirus 71 type 3C protease
[0022] The DNA sequence encoding enterovirus 71 type 3C protease was artificially synthesized and ligated into the pET-28a vector with double restriction sites. Transform Escherichia coli BL21(DE3) competent cells with the recombinant plasmids, pick the obtained transformant clones and transfer them to an appropriate amount of LB medium (add kanamycin to a final concentration of 50 μg / mL), and culture at 37°C to 600nm The absorbance was 0.8, adding isopropyl-β-D-thiogalactopyranoside (IPTG) inducer to a final concentration of 0.5mmol / L, and induced at 20°C for 12h. After collecting the bacteria by centrifugation at 4200r / min for 30min, suspend the bacteria with an appropriate amount of buffer (25mmol / L Tris-HCl (trishydroxymethylaminomethane-hydrochloric acid), pH7.5, 150mmol / L sodium chloride). The bacteria were lysed by sonication, and centrifuged at 16000r / min for 40min to remove sediment and...
Embodiment 2
[0023] Example 2 In vitro activity detection of enterovirus 71 type 3C protease
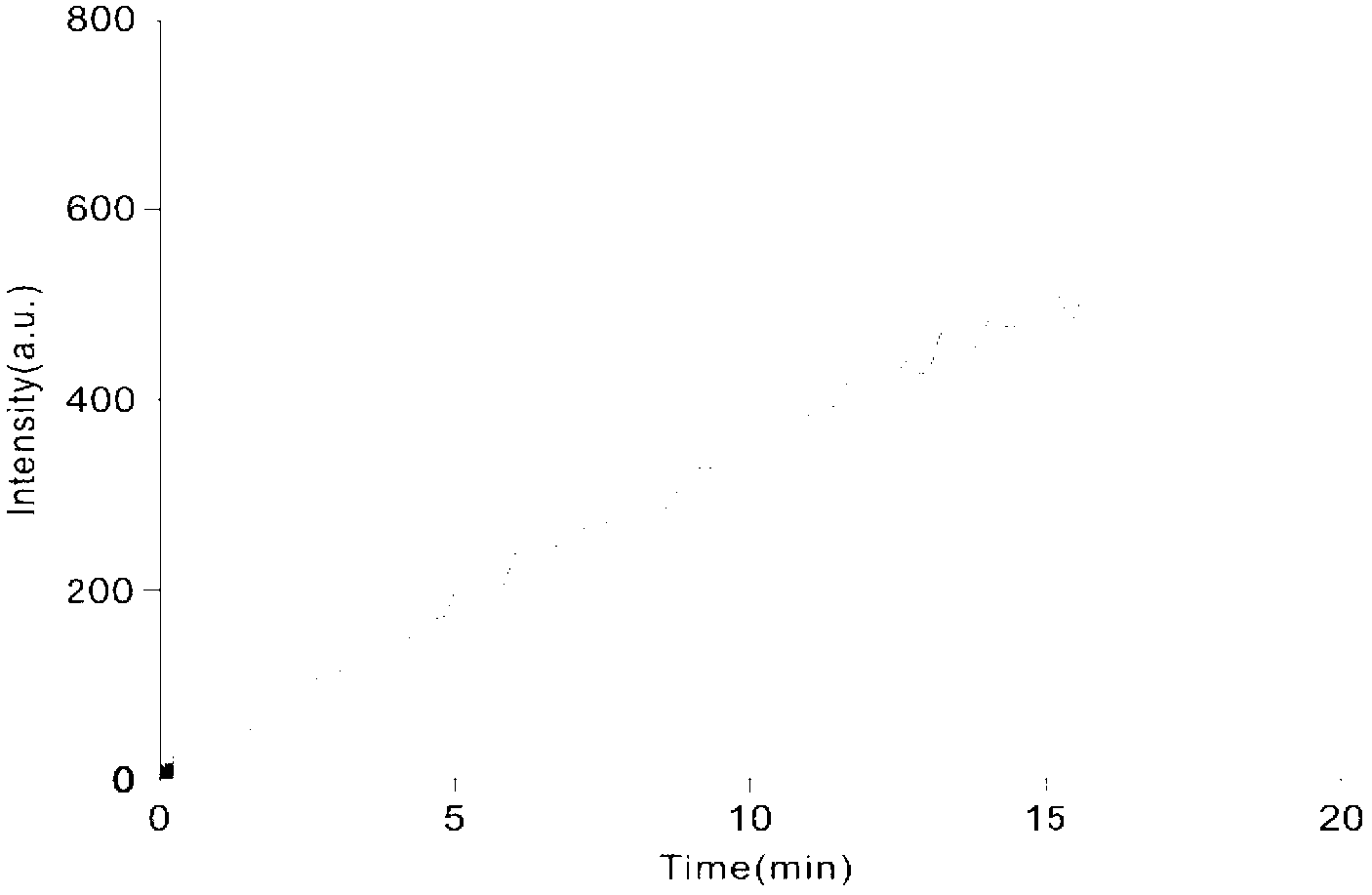
[0024] Use a fluorescence spectrophotometer to test the enzyme activity. The sequence of the fluorescent substrate polypeptide is Dacyl-KRTATVQGPSLDFE-Edans. The total volume of the reaction system is 100μL, and the buffer is 50mmol / LTris-HCl, pH7.0, 100mmol / L sodium chloride , 1mmol / L Tri-β-chloroethyl phosphate (TCEP), the final protein concentration was 5μmol / L, and the substrate concentration was 50μmol / L. Excited with light at 340nm, the fluorescence at 495nm was detected to gradually increase, the results are as follows figure 1 As shown, it is known that the polypeptide substrate is cleaved.
Embodiment 3
[0025] Example 3 Crystallization and structural analysis of enterovirus 71 type 3C protease
[0026] 3.1. Crystallization conditions:
[0027] Crystallization was carried out by the hanging drop method, the volume ratio of protein solution and crystallization reagent in the crystallization droplet was 1:1, the protein concentration in the protein solution was 12mg / mL, and the crystallization conditions were 100mmol Tris-HCl pH8.5, 25% polyethylene glycol 4000, 0.8mol lithium chloride, the crystals are stored at 16°C.
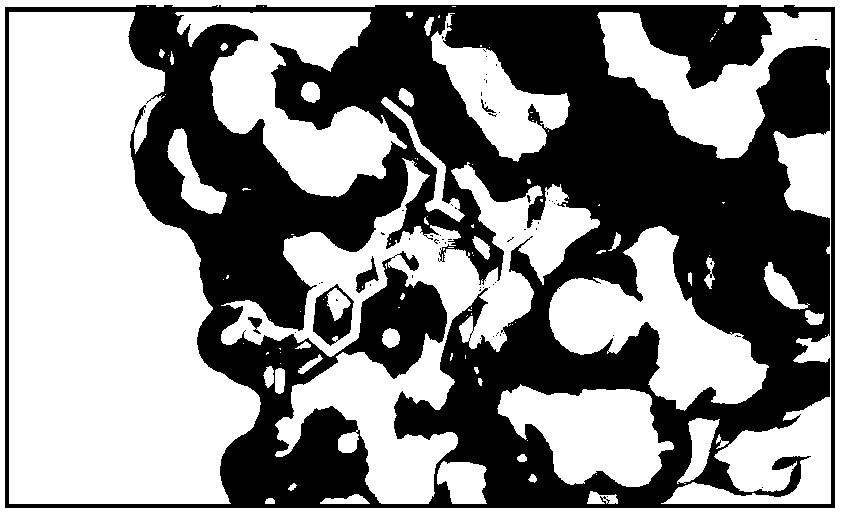
[0028] In order to obtain the complex formed by the 3C protease and the inhibitor molecule, the protease and the compound were mixed in a molar ratio of 1:5 at 4° C. overnight. The conditions for co-crystallization of protease and compound are the same as when no compound is added.
[0029] 3.2. Data collection and structure analysis:
[0030] Data collection uses SSRF synchrotron radiation, and data processing uses HKL2000 ([5] Z.Otwinowski and W.Minor, "Proce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com