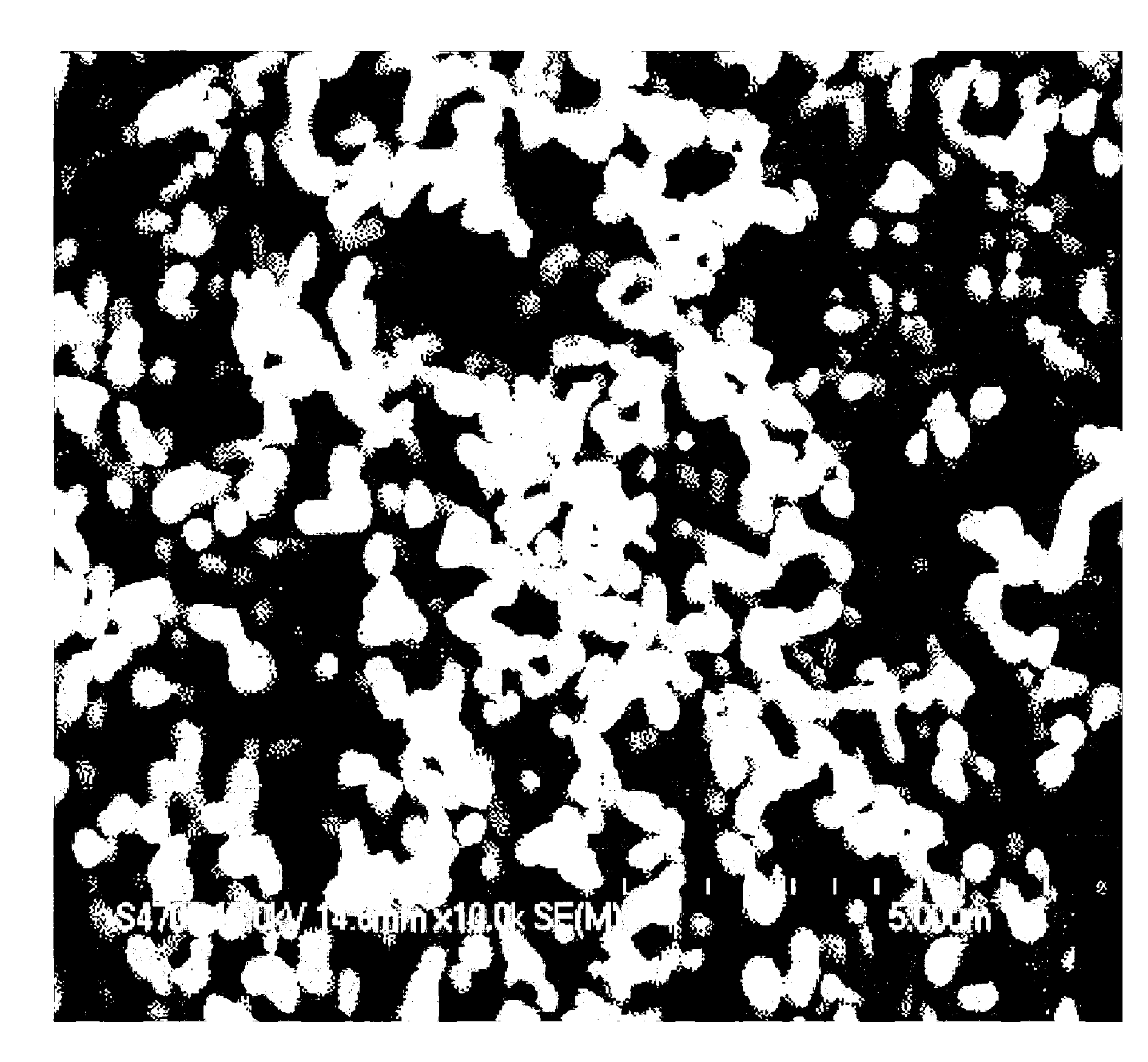
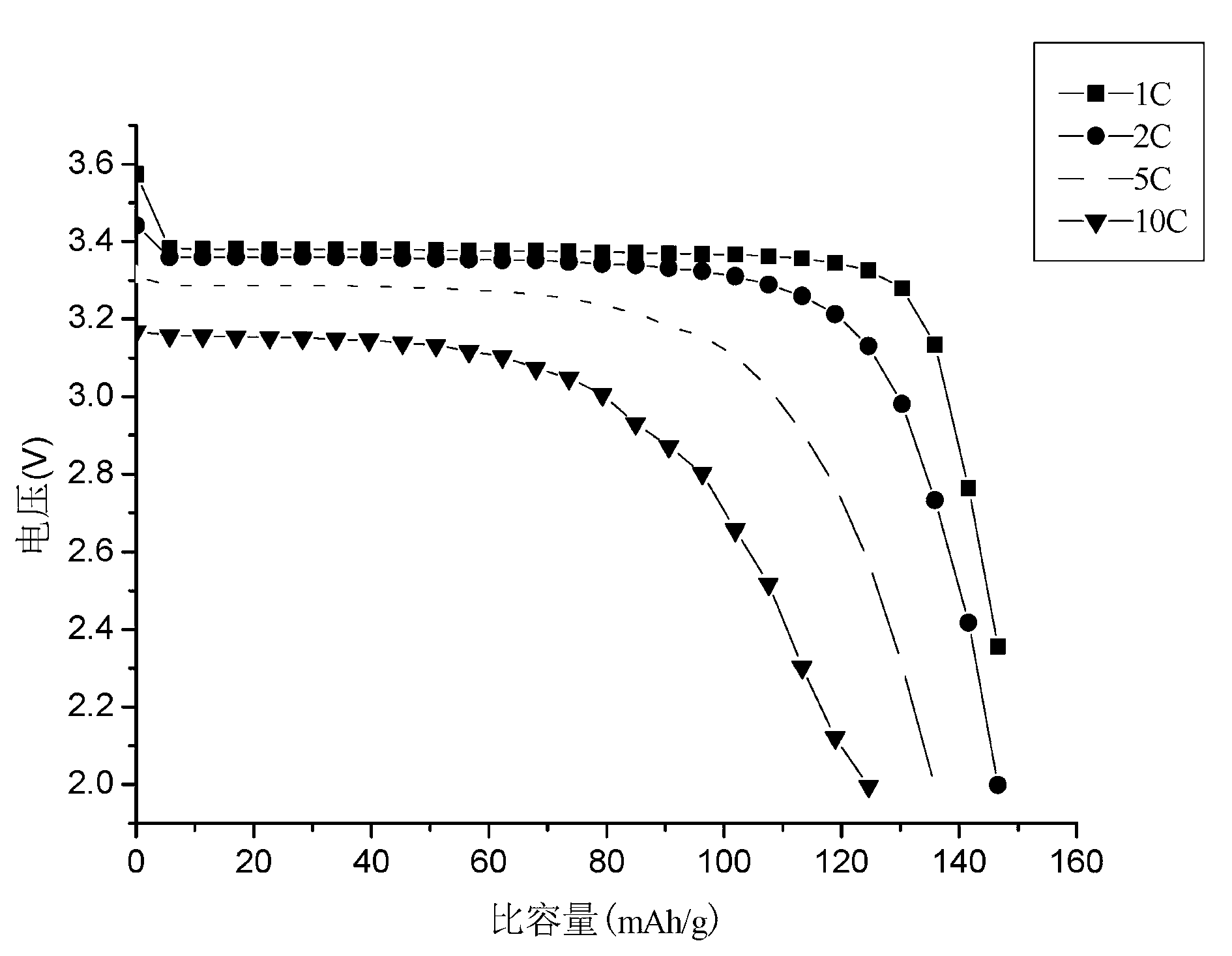
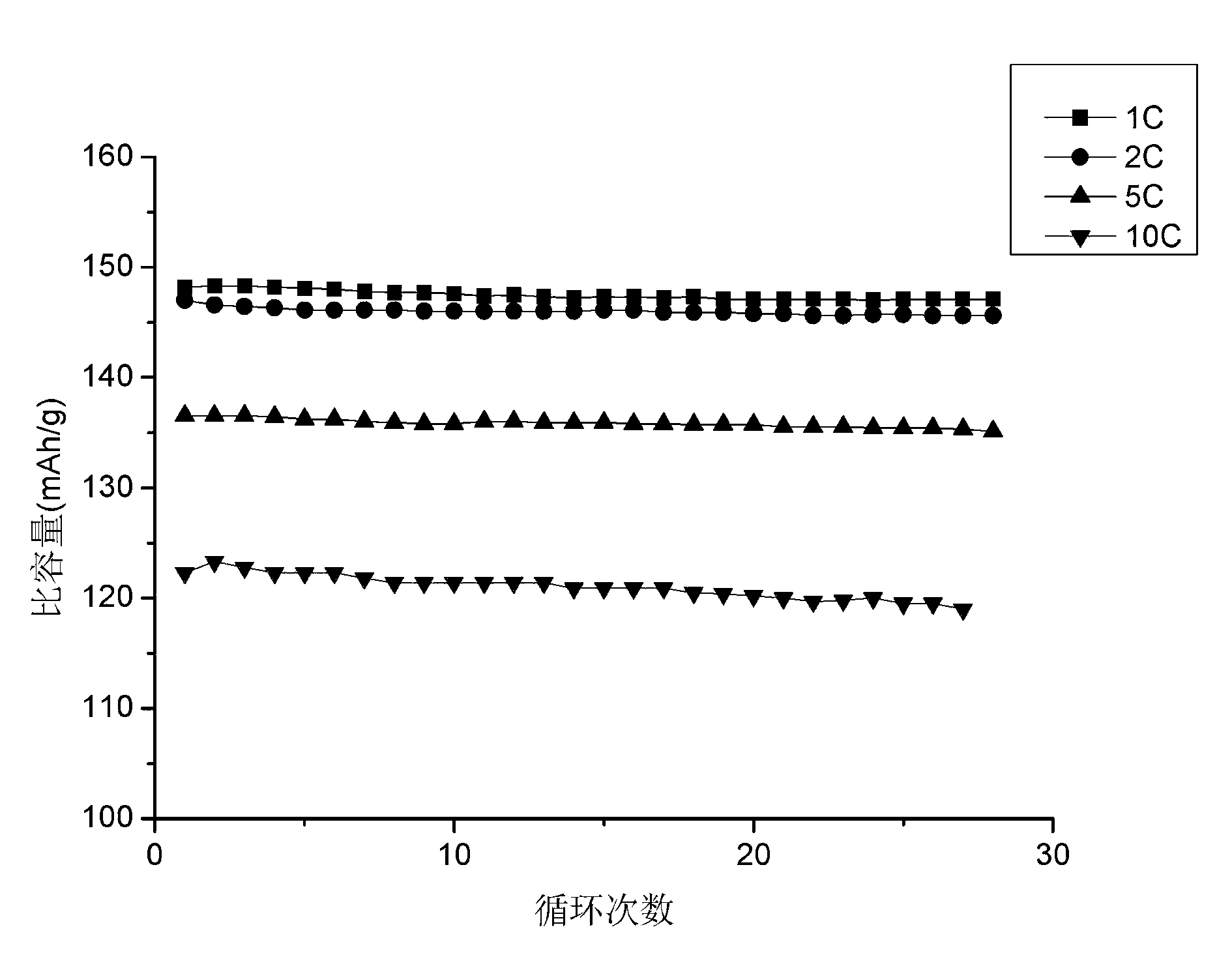
Method for preparing lithium iron phosphate in batch-type high-vacuum dynamic sintering mode
A lithium iron phosphate, high vacuum technology, applied in chemical instruments and methods, phosphorus compounds, structural parts, etc., can solve the problems of increasing uncontrollable factors, slow cooling speed, uneven heating, etc., to shorten the sintering time and reduce sintering. temperature, the effect of preventing oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: In this embodiment, according to the stoichiometric ratio Fe:Li:P=1:1:1, ferric phosphate, lithium carbonate and organic carbon source (in this embodiment, the weight percent of organic carbon source accounts for all precursors 1%~5% of the body) as the raw material to prepare the precursor. The lithium iron phosphate precursor is sent into the rotary furnace body, and the furnace head and the rotation are sealed with a gasket and a high-temperature-resistant vacuum grease to ensure that the furnace head and rotation Then adjust the level of the furnace body, turn on the multi-stage vacuum pump to extract the residual air in the furnace body, and then fill it with inert gas. After repeated several times, turn on the power supply of the rotary furnace and adjust the rotation speed of the furnace body to 0.5 Turn / min, material at 10 2 ~10 -2 Raise the temperature to 700°C under high vacuum for 8 hours, turn off the vacuum pump, and then fill in inert gas to...
Embodiment 2
[0039] Embodiment 2: in the present embodiment, be Fe:Li:P=1:1:1 ferrous oxalate, lithium carbonate, ammonium dihydrogen phosphate with stoichiometric ratio as raw material, in the present embodiment, also include weight percent The 1%~5% organic carbon source of all precursors is obtained by sand grinding and drying to obtain the precursor of lithium iron phosphate, and then the precursor is sent into the rotary furnace body, and the furnace head and the rotary Carry out sealing treatment at the furnace head, the rotating part and the gas outlet pipe, then adjust the level of the furnace body, turn on the secondary vacuum pump to extract the residual air in the furnace body, and then fill it with nitrogen gas, and then turn on the back door after repeated times. Converter power supply, adjust the rotation speed of the furnace body to 5 revolutions / min, and the material is at 10 2 ~10 -2 Raise the temperature to 600°C under high vacuum for 15 hours, turn off the vacuum pump, ...
Embodiment 3
[0041] Example 3: In this example, iron oxide, lithium carbonate, and phosphoric acid with a stoichiometric ratio of Fe:Li:P=1:1:1 are used as raw materials. In this example, the percentage by weight of all precursors is also included. 1%~5% organic carbon source, the lithium iron phosphate precursor is obtained by ball milling and spray drying, and then the precursor is sent into the rotary furnace body, and the furnace head and the rotation are sealed with gaskets and high-temperature-resistant vacuum grease , to ensure the airtightness of the furnace head, the rotating part and the gas outlet, then adjust the level of the furnace body, turn on the four-stage vacuum pump to extract the residual air in the furnace body, and then fill it with argon gas, and turn on the power of the rotary furnace after repeated times. Adjust the rotation speed of the furnace body to be 1 revolution / min, and the material is at 10 2 ~10 -2 Raise the temperature to 800°C under high vacuum for 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com