Electrochemical preparation method for ZnS nanocrystalline semiconductor precursor film or semiconductor film
A nanocrystal and nanocrystal technology is applied in the field of electrochemical preparation of ZnS nanocrystal semiconductor thin films, which can solve the problems of turbid solution, lack of preferred orientation of ZnS nanocrystal thin films, uneven solution concentration and temperature distribution, etc. Uniform and stable growth, enhanced controllability and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Weigh 0.878g Zn(CH 3 COO) 2 2H 2 O, 0.993g Na 2 S 2 o 3 ·5H 2 O, 0.01g C 7 h 6 o 6 S·2H 2 O, 0.0075g Na 2 SO 3 (The reagents used are all analytical grade, the molar ratio is 20:20:0.2:0.3) dissolved in 2.416g LiCl·H 2 O in 200ml aqueous solution, and adjust pH=3.5 with dilute sulfuric acid, stir well.
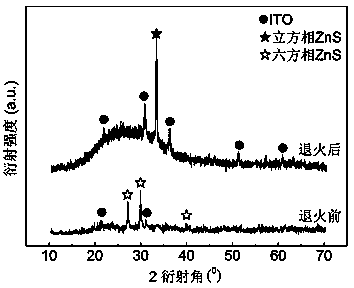
[0031] 2) Use the solution prepared in step 1) as the electrolyte, use the pretreated ITO conductive glass as the working electrode (cathode), and the platinum sheet as the counter electrode (anode), and use wires to connect the two to the potentiostat respectively. On the working electrode and auxiliary electrode terminal button, ensure that the distance between ITO and platinum sheet is 5 cm, use the temperature control device to control the temperature of the electrolyte at 30 ± 1 ℃, and control the current density at 10 mA cm -2 , the electrodeposition time was controlled at 10 min, and a pure hexagonal phase ZnS precursor film was obtained.
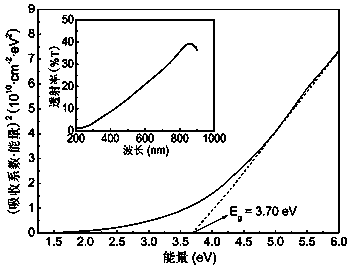
[0032...
Embodiment 2
[0034] 1) Weigh 0.878g Zn(CH 3 COO) 2 2H 2 O, 0.993g Na 2 S 2 o 3 ·5H 2 O, (0.01 or 0.02 or 0.04) g C 7 h 6 o 6 S·2H 2 O, 0.01g Na 2 SO 3 (The reagents used are of analytical grade, the molar ratio is 20:20:(0.2 or 0.4 or 0.8):0.4), dissolved in three cups containing 1.208g LiCl·H 2 O in 200ml aqueous solution, and adjust the pH=3.5 with dilute sulfuric acid, and stir well.
[0035] 2) Use the 3 cups of solution prepared in step 1) as the electrodeposition solution, the pretreated ITO conductive glass as the working electrode (cathode), the platinum sheet as the counter electrode (anode), and the saturated calomel as the reference electrode, And use wires to connect the three electrodes to the working electrode, auxiliary electrode and reference electrode of the potentiostat respectively to ensure that the distance between the ITO and the platinum sheet is 5 cm, and use the temperature control device to control the temperature of the electrodeposition solution at 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com