Production technology of ammonium bifluoride
A technology of ammonium bifluoride and production process, applied in the direction of ammonium halide, etc., can solve the problem of high cost of hydrofluoric acid, achieve the effects of reducing raw material costs, improving product quality, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
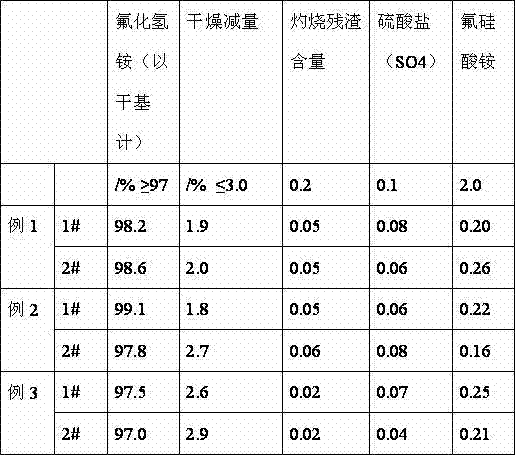
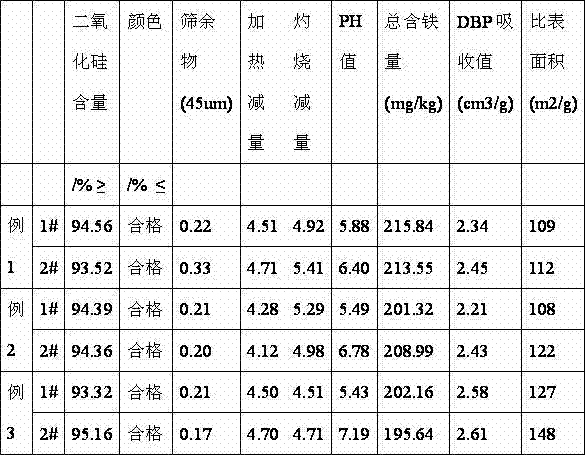
Embodiment 1
[0025] 1. Add fluosilicic acid, a by-product of the anhydrous hydrogen fluoride production line, with a mass percentage concentration of 20% into the reactor, start stirring, and add sodium nitrilotriacetate at a uniform speed according to the required moles of the reaction formula Add 1.1 times of the mass percent concentration of ammonia water of 25%, fully react, and the pH value of the reaction end point is 8.5-9.
[0026] 2. After the reaction in the reactor is complete, the reaction liquid is separated from the solid and liquid, and the solid is beaten and cleaned to become high-purity silica, which can be sold as white carbon black after drying.
[0027] 3. Add the reaction liquid into the transfer tank, settle for 24 hours, and pour the clear liquid into the deamination kettle.
[0028] 4. Heating the reaction solution to remove ammonia, and the ammonia gas generated by the reaction is absorbed into ammonia water by cooling cycle, and stored in a storage tank for futur...
Embodiment 2
[0032] 1. Add fluosilicic acid, a by-product of the anhydrous hydrogen fluoride production line, with a mass percentage concentration of 30% into the reaction kettle, start stirring, add sodium nitrilotriacetate at a rate of 0.2% of the weight of fluosilicic acid, and uniformly follow the required moles of the reaction formula Add 1.1 times of the mass percent concentration of ammonia water of 25%, fully react, and the pH value of the reaction end point is 8.5-9.
[0033] 2. After the reaction in the reactor is complete, the reaction liquid is separated from the solid and liquid, and the solid is beaten and cleaned to become high-purity silica, which can be sold as white carbon black after drying.
[0034] 3. Add the reaction liquid into the transfer tank, settle for 24 hours, and pour the clear liquid into the deamination kettle.
[0035] 4. Heating the reaction solution to remove ammonia, and the ammonia gas generated by the reaction is absorbed into ammonia water by cooling...
Embodiment 3
[0039] 1. Add fluosilicic acid, a by-product of anhydrous hydrogen fluoride production line, with a mass percentage concentration of 40% into the reaction kettle, start stirring, add sodium nitrilotriacetate in an amount of 0.2% of the weight of fluosilicic acid, and uniformly follow the required moles of the reaction formula Add 1.1 times of the mass percent concentration of ammonia water of 25%, fully react, and the pH value of the reaction end point is 8.5-9.
[0040] 2. After the reaction in the reactor is complete, the reaction liquid is separated from the solid and liquid, and the solid is beaten and cleaned to become high-purity silica, which can be sold as white carbon black after drying.
[0041] 3. Add the reaction liquid into the transfer tank, settle for 24 hours, and pour the clear liquid into the deamination kettle.
[0042] 4. Heating the reaction solution to remove ammonia, and the ammonia gas generated by the reaction is absorbed into ammonia water by cooling ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com