Purification method in preparation of methicillin staphylococcus aureus-resistant recombinant genetic engineering vaccine candidate antigen I12C
A genetically engineered vaccine and methicillin-resistant technology, applied in the field of biotechnology and pharmaceuticals, can solve the problems of unfavorable vaccine industrial preparation, cumbersome methods, and low content, and achieve the effects of clear vaccine ingredients, controllable quality, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
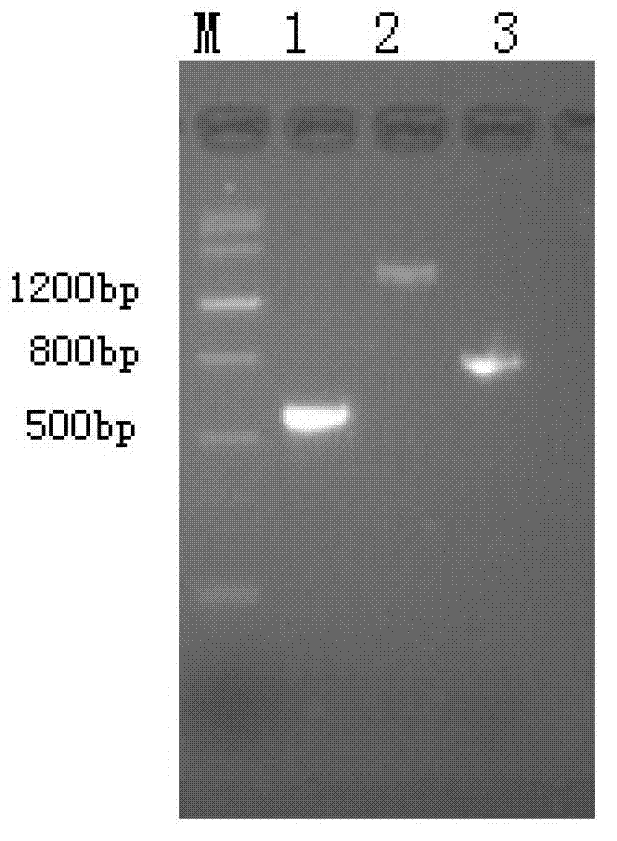
[0054] Example 1: Expression I 12 Construction of C engineering bacteria
[0055] Antigen I 12 The nucleotide sequence of C is shown in SEQ ID NO:1, and the amino acid sequence of its protein is shown in SEQ ID NO:2.
[0056] 1. Take out the MRSA-252 strain of methicillin-resistant Staphylococcus aureus from the freezer at -80°C and spread it on the special solid medium for MRSA-252, cultivate it overnight at 37°C, and then pick a single colony and inoculate it on MRSA- 252 special liquid culture medium for 8 hours, referring to the bacterial genome extraction kit (Shanghai Sangong) to extract the MRSA genome.
[0057] 2. Using the PCR method to self-synthesize template I 12 Amplification I 12 -Linker-gene fragment, the amplification steps are as follows:
[0058] 1) Design PCR primers P1 and P2, which are SEQ ID NO: 9-10, respectively, among them, P1 (5'-GCGGATCCATGGGCAGCGCACCAAACTCTCG-3') and P2 (5'-GCTTCTTTACTGCTGCTGCCACCGCCACCGGCATTGGCTTTAGTAAA-3').
[0059] 2) Using...
Embodiment 2
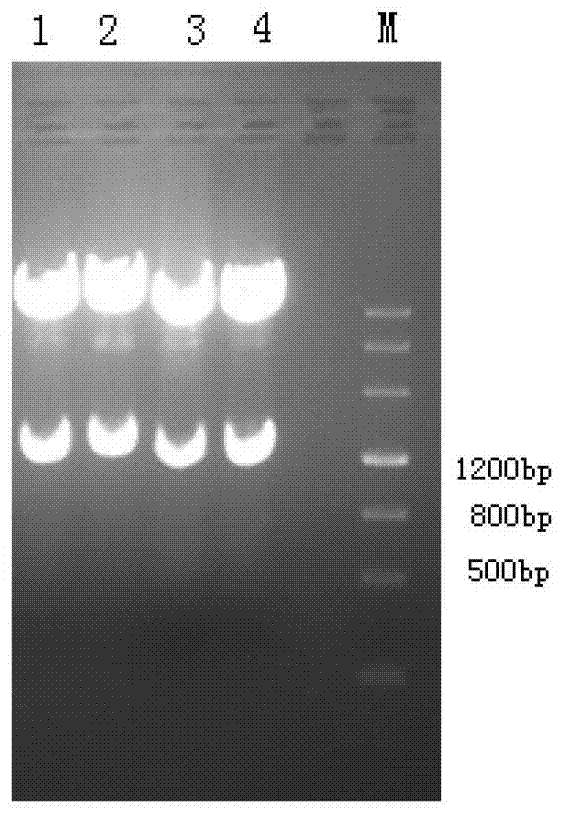
[0100] Example 2: Expression I 12 High-density fermentation of C engineering bacteria.
[0101] 1) Recovery, activation and identification of MRSA vaccine engineered bacteria for fermentation
[0102] (1) Recovery of engineered strains of MRSA vaccine
[0103] Take 100 μl of strains preserved at -70°C in 20% glycerol, inoculate them on a plate containing A+LB solid medium, and incubate overnight at 37°C. After the colonies grow, store them at 4°C.
[0104] (2) Activation of seed bacteria
[0105] Pick a single colony with uniform shape and size and inoculate it in a medicine bottle containing 5ml of A+LB medium, culture at 37°C and 200r / min for 5-7h, and the OD600 reaches 0.6-0.8, become activated bacteria, and store at 4°C.
[0106] (3) Identification of seed fungus
[0107] The activated bacteria were taken for morphological detection, Gram staining detection, antibiotic resistance detection, biochemical reaction detection, and bacterial species identification.
[0108...
Embodiment 3
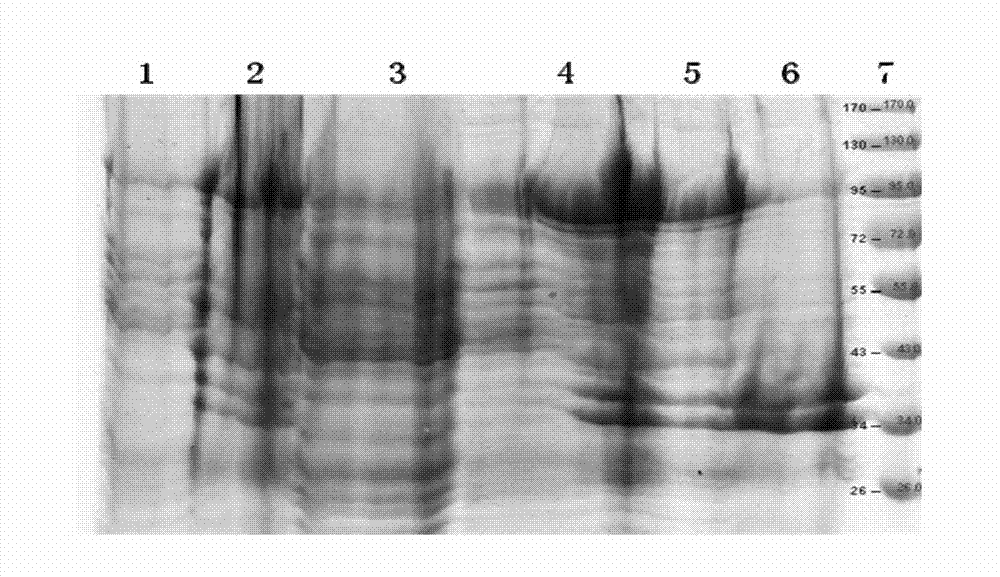
[0124] Example 3: Expression I 12 Autoclaving and centrifugation of C engineering bacteria
[0125] The recombinant double-subunit genetic engineering protein I constructed by the applicant to express soluble methicillin-resistant Staphylococcus aureus 12 The Escherichia coli engineering bacteria of C, through high-density fermentation, the expression rate of the target protein is 13%, and the bacteria are collected by centrifugation for later use.
[0126] 200-500 g of bacterial cells were mixed with PBS (10-20 mM, pH 7.0-7.5) buffer solution according to the weight: volume ratio of 1:10, and pre-cooled at 4°C.
[0127] High-pressure homogenizer: Use distilled water to flush the pipeline of the high-pressure homogenizer, and turn on the low-temperature circulation system to pre-cool to 1-4°C for later use.
[0128] Add the pre-cooled suspended bacteria liquid to a high-pressure homogenizer, maintain the pressure at 60-80Mpa to break the bacteria 3-5 times, take a smear of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com