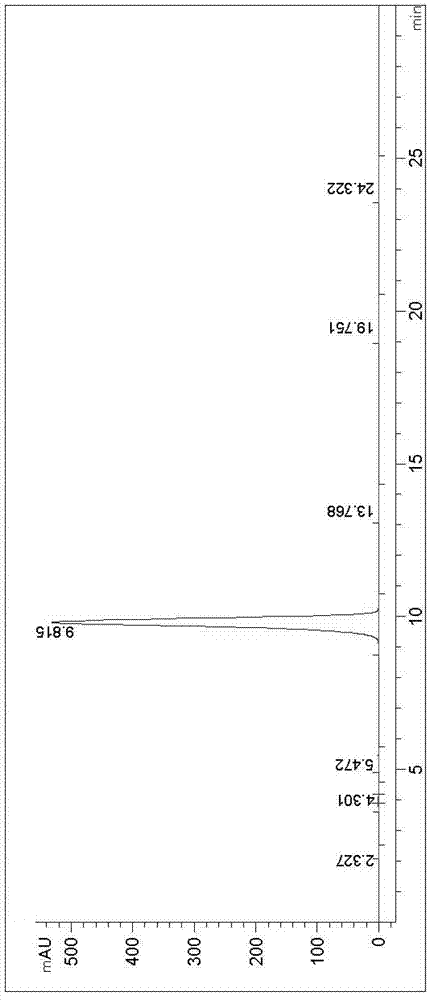
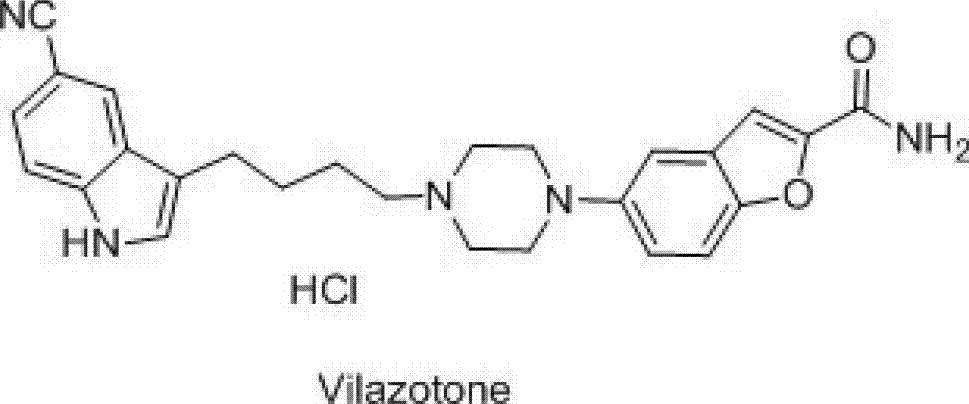
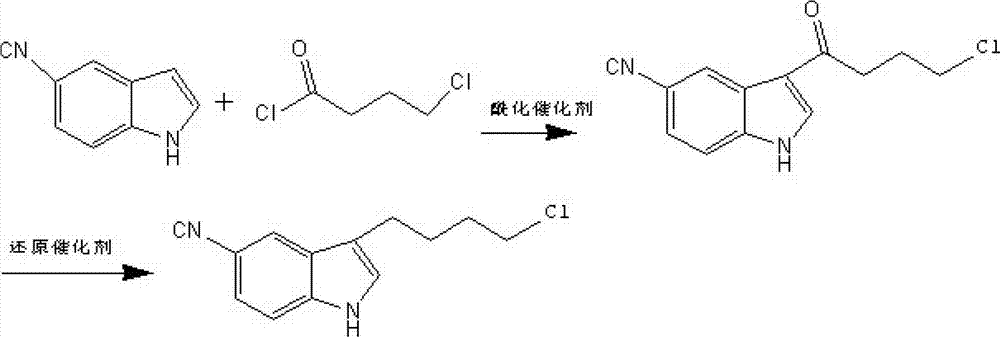
New synthesis method of 3-(4-chlorobutyl)-5-cyanoindole
A cyanoindole and a synthesis method technology are applied in the synthesis of pharmaceutical intermediates, and the new synthesis field of vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-5-cyanoindole can solve the problem of Human health hazards, strong corrosiveness, easy deliquescence of aluminum, etc., to achieve the effects of good market competitiveness, good quality, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] At room temperature, add 500ml of methanol to the reaction bottle, then add 50g of cycloheptene, cool the mixed solution to -30°C, then pass in oxygen-ozone to saturate the reaction solution, then slowly raise the temperature to 40°C, and react After 15 minutes, cool down and replace excess oxygen and ozone in the reaction solution. Add this reaction solution dropwise to the reflux solution of ferric chloride hexahydrate methanol, continue to react for 15 minutes after the dropwise addition, cool to room temperature, extract once with light petroleum ether, dilute the reaction solution with water, and then dichloromethane Extract once. The solution was distilled off to give 1,1-dimethoxy-6-chlorohexane as an oil.
[0032] At room temperature, add 100ml of ethanol to the reaction bottle under the protection of nitrogen, then add 50ml of pure water, then add 18.1g of the 1.1-dimethoxy-6-chlorohexane prepared above, and raise the temperature to 68°C until 1.1-dimethyl Ox...
Embodiment 2
[0035] At room temperature, add 500ml of methanol to the reaction bottle, then add 50g of cycloheptene, cool the mixed solution to -30°C, then pass in oxygen-ozone to saturate the reaction solution, then slowly raise the temperature to 40°C, and react After 15 minutes, cool down and replace excess oxygen and ozone in the reaction solution. Add this reaction solution dropwise to the reflux solution of ferric chloride hexahydrate methanol, continue to react for 15 minutes after the dropwise addition, cool to room temperature, extract once with light petroleum ether, dilute the reaction solution with water, and then dichloromethane Extract once. The solution was distilled off to give 1,1-dimethoxy-6-chlorohexane as an oil.
[0036]At room temperature, add 80ml of methanol to the reaction bottle under the protection of nitrogen, then add 54ml of pure water, then add 20.5g of the 1.1-dimethoxy-6-chlorohexane prepared above, and raise the temperature to 70°C until 1.1-dimethyl Oxy...
Embodiment 3
[0039] At room temperature, add 500ml of methanol to the reaction bottle, then add 50g of cycloheptene, cool the mixed solution to -30°C, then pass in oxygen-ozone to saturate the reaction solution, then slowly raise the temperature to 40°C, and react After 15 minutes, cool down and replace excess oxygen and ozone in the reaction solution. Add this reaction solution dropwise to the reflux solution of ferric chloride hexahydrate methanol, continue to react for 15 minutes after the dropwise addition, cool to room temperature, extract once with light petroleum ether, dilute the reaction solution with water, and then dichloromethane Extract once. The solution was distilled off to give 1,1-dimethoxy-6-chlorohexane as an oil.
[0040] At room temperature, add 158ml of ethanol to the reaction bottle under the protection of nitrogen, then add 70ml of pure water, then add 25.7g of the 1.1-dimethoxy-6-chlorohexane prepared above, and raise the temperature to 69°C until 1.1-dimethyl Ox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com