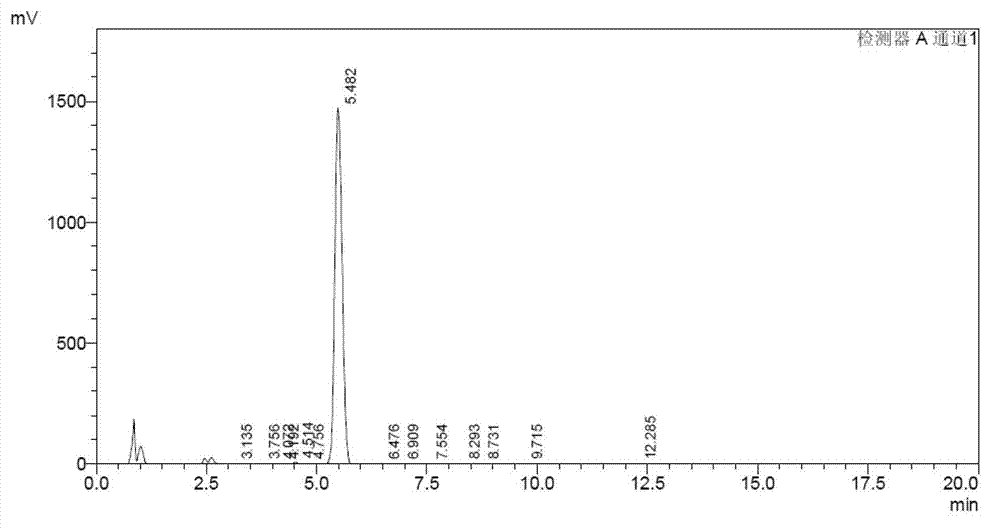
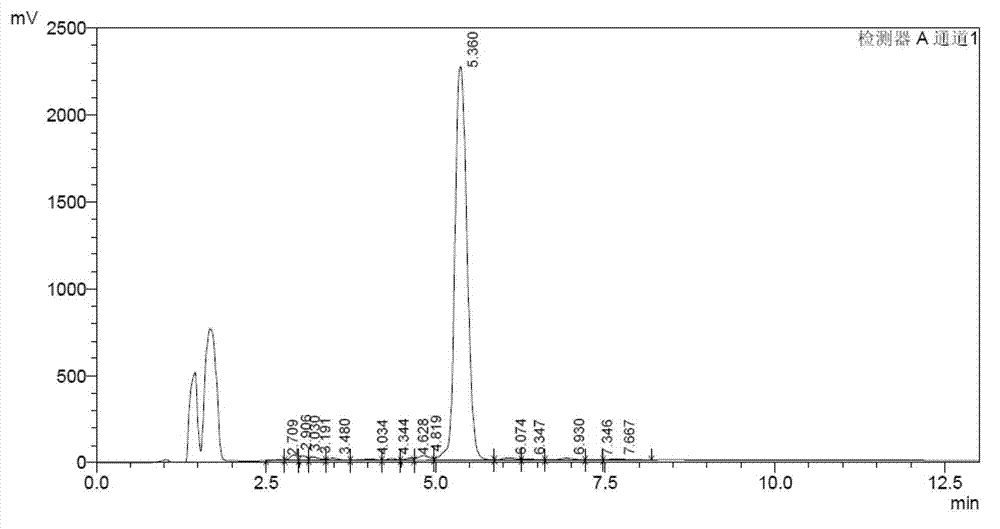
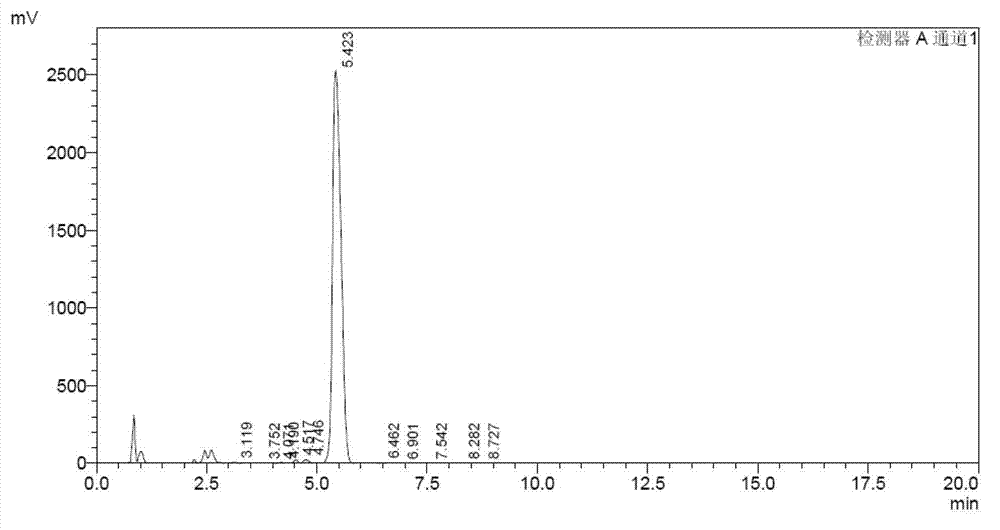
Production process of Lipstatin
A production process, lipox technology, applied in the field of drug synthesis, can solve the problems of low fermentation titer and high production cost, and achieve the effect of low fermentation cost, low cost and short fermentation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 A production process of riprestatin
[0037] Including the following steps:
[0038] A. Preparation of fermentation medium
[0039] In terms of mass percentage, the formula of 20L fermentation medium is: 2.5% glycerin, 3% soybean powder, 1.5% lecithin, 5% sunflower oil, 0.01% magnesium sulfate, 0.05% calcium carbonate, appropriate amount of trace elements (chlorinated Manganese: 0.001%, zinc chloride 0.0015%), and the balance is deionized water. Sterilize the culture medium by conventional methods.
[0040] B. Inoculation, fermentation culture
[0041] Streptomyces toxin was inoculated in the fermentation medium, the inoculum amount was 5% (V / V) of the fermentation broth, and the strain concentration of the bacteria solution was 15%; fermented at 27°C±1°C for 8 days; using hydrogen oxidation Sodium and hydrochloric acid are automatically balanced to adjust the pH value of the fermentation broth, so that the pH value is maintained at 7.0; during the ferment...
Embodiment 2
[0051] Embodiment 2 A kind of production process of riprestatin
[0052] Including the following steps:
[0053] A. Preparation of fermentation medium
[0054] In terms of mass percentage, the formula of 20L fermentation medium is: 3% glycerin, 4.5% soybean powder, 2% lecithin, 8% sunflower oil, 0.05% magnesium sulfate, 0.2% calcium carbonate, appropriate amount of trace elements (chloride Manganese: 0.001%, zinc chloride 0.0015%), and the balance is deionized water. Sterilize the culture medium by conventional methods.
[0055] B. Inoculation, fermentation culture
[0056]Streptomyces toxin was inoculated in the fermentation medium, the inoculation amount was 8% (V / V) of the fermentation broth, and the strain concentration of the bacteria solution was 20%; fermented at 27°C±1°C for 7 days; using hydrogen oxidation Sodium and hydrochloric acid are automatically balanced to adjust the pH value of the fermentation broth, so that the pH value is maintained at 7.0; during the ...
Embodiment 3
[0066] Example 3 A production process of riprestatin
[0067] Including the following steps:
[0068] A. Preparation of fermentation medium
[0069] In terms of mass percentage, the formula of 20L fermentation medium is: 4.5% glycerin, 6.5% soybean powder, 4% lecithin, 9% sunflower oil, 0.15% magnesium sulfate, 0.4% calcium carbonate, appropriate amount of trace elements (chloride Manganese: 0.001%, zinc chloride 0.0015%), and the balance is deionized water. Sterilize the culture medium by conventional methods.
[0070] B. Inoculation, fermentation culture
[0071] Streptomyces toxin was inoculated in the fermentation medium, the inoculation amount was 10% (V / V) of the fermentation broth, and the strain concentration of the bacteria solution was 20%; fermented at 27°C±1°C for 8 days; using hydrogen oxidation Sodium and hydrochloric acid are automatically balanced to adjust the pH value of the fermentation broth, so that the pH value is maintained at 7.0; during the ferment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com