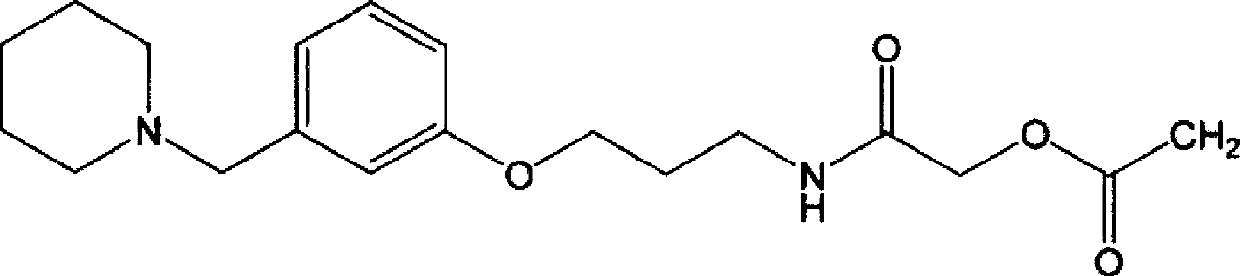
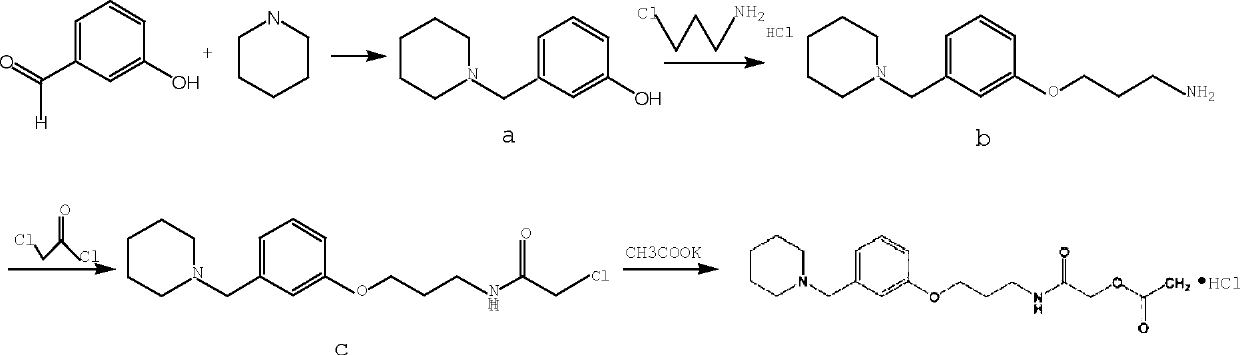
Synthetic method for preparing roxatidine acetate hydrochloride with high purity
A technology for roxatidine acetate and hydrochloride, which is applied in the field of synthesis and preparation of high-purity roxatidine acetate hydrochloride, can solve the problem of low product conversion rate and difficulty in obtaining high-purity roxatidine acetate , the synthesis method is complicated to operate, etc., to achieve the effect of high product purity, conducive to industrial development and application, simple and safe synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 3-(1-piperidinylmethyl)phenol
[0028] Place a 1000ml three-necked bottle in a low-temperature reaction tank, install and stir, add 70g of m-hydroxybenzaldehyde into the three-necked bottle while stirring, add 500ml of absolute ethanol after the addition, dissolve slowly under stirring, lower the temperature to 5°C, and slowly add six Hydropyridine 127ml, the solution becomes light yellow, and there is exothermic phenomenon, continue to stir for 30min to make it completely dissolved; control the temperature below 15°C and slowly add 23.8g of sodium borohydride in 6 times, there is exotherm during the addition, accompanied by Gas was generated. After the feeding was completed, the low-temperature reaction tank was closed, and the stirring reaction was continued for 5 hours at room temperature. (TLC monitors until complete, developer: dichloromethane / methanol=5:1) After the reaction is completed, concentrate under reduced pressure and distill off the reagent;...
Embodiment 2
[0030] Synthesis of 3-(3-(1-piperidinylmethyl)phenoxy)propylamine
[0031] Add 60g of the product intermediate a of the previous step and 350ml of anhydrous DMF into a 1000ml three-necked bottle, dissolve them all under stirring, slowly add 5.8g of sodium hydride and 170g of sodium hydroxide, and gas is generated; add 51g of 3-chloropropylamine hydrochloride under stirring, Introduce nitrogen, under the protection of nitrogen, heat up to 90-95°C and react for 2 hours. After the reaction, cool to room temperature, filter to remove the solid, collect the filtrate, wash the filter cake with DMF for 3 times, and concentrate the filtrate under reduced pressure to remove the reagent, leaving a reddish-brown liquid Add 400ml of ice water to the mixture, adjust the pH to 5 with glacial acetic acid, add 150ml of dichloromethane to extract and wash, add concentrated ammonia water to adjust the pH to 9, and generate white turbidity, extract with 150ml of dichloromethane×2 times, discard ...
Embodiment 3
[0033] Synthesis of 2-chloro-N-[3-[3-(piperidin-1-ylmethyl)phenoxy]propyl]acetamide
[0034] Dissolve 50g of the product intermediate (b) from the previous step in 200ml of dichloromethane, add 48.4g of anhydrous potassium carbonate while stirring, open the low-temperature reaction tank to control the internal temperature below 3°C, slowly add 28.4g of chloroacetyl chloride dropwise, drop After the addition, keep the internal temperature at 0-5°C and react for 3 hours at a low temperature. After the reaction, add 120ml of ice water, extract and separate the layers, discard the water layer; add 100ml of saturated saline to wash twice, anhydrous sulfuric acid Sodium was dried and filtered, and the filtrate was concentrated by distillation under reduced pressure to obtain a white oily product (c), weighing 61g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com