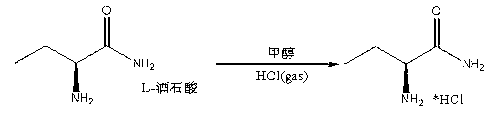Synthesis method of S-2-aminobutanamide hydrochloride
A technology of aminobutyramide and S-2-, which is applied in the field of organic chemical synthesis and preparation, can solve problems such as poor absorption of sulfur dioxide, hidden dangers of production safety, and increased production costs, and achieve low equipment requirements, increased yield, and mild method conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Into a 2000ml reaction bottle, add 1350g of methanolic ammonia solution containing 25% ammonia by weight, take a sample for inspection to ensure that the ammonia content is 25%, lower the temperature below 10°C, and add 300g of 2-bromine dropwise within 2 hours Methyl butyrate, after dripping methyl 2-bromobutyrate, close the reaction vessel, keep it warm for 1 hour, remove the freezer, raise the temperature naturally to 20°C, stir for 40 hours, during the heat preservation process, take a sample to detect methyl 2-bromobutyrate , when the content is less than 0.5%, reach the end of the reaction, evaporate to dryness under reduced pressure at 75°C to obtain a white solid, ammonia water can be recycled in this process, add 750g of anhydrous isopropanol to the obtained white solid, reflux for 2 hours, and then freeze To 10°C, shake filter, the white solid obtained by the shake filter is ammonium bromide for solid waste treatment, the mother liquor obtained by the shake fil...
Embodiment 2
[0026] Into a 2000ml reaction bottle, add 1350g of methanolic ammonia solution containing 25% ammonia by weight, take a sample for inspection to ensure that the ammonia content is 25%, lower the temperature below 10°C, and add 300g of 2-bromine dropwise within 3 hours Methyl butyrate, after dripping methyl 2-bromobutyrate, close the reaction vessel, keep it warm for 1 hour, remove the freezer, raise the temperature naturally to 20°C, stir for 38 hours, during the heat preservation process, take a sample to detect methyl 2-bromobutyrate , when the content is less than 0.5%, reach the end of the reaction, evaporate to dryness under reduced pressure at 60°C to obtain a white solid, ammonia water can be recycled in this process, add 800g of anhydrous isopropanol to the obtained white solid, reflux for 2 hours, and then freeze To 10°C, shake filter, the white solid obtained by the shake filter is ammonium bromide for solid waste treatment, the mother liquor obtained by the shake fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com