Preparation method of pemetrexed or pemetrexed salt
A technology for pemetrexed and a compound is applied in the field of high-yield preparation of pemetrexed and its salts, and can solve the problems of unsatisfactory effect of pyrrole ring closing reaction, difficult reaction control, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
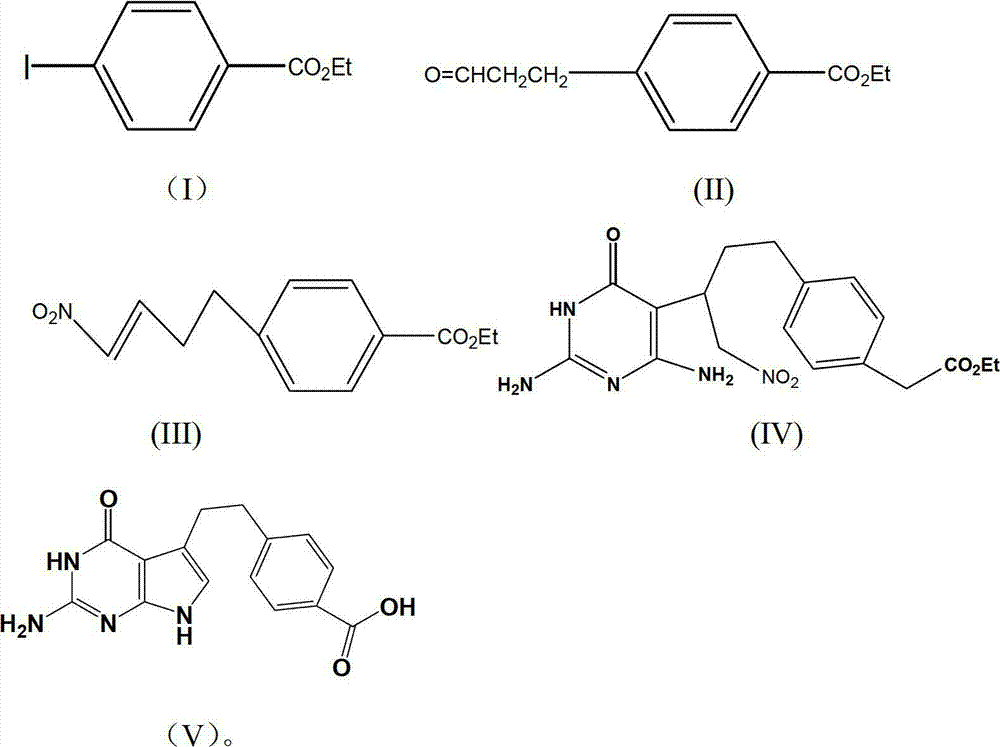
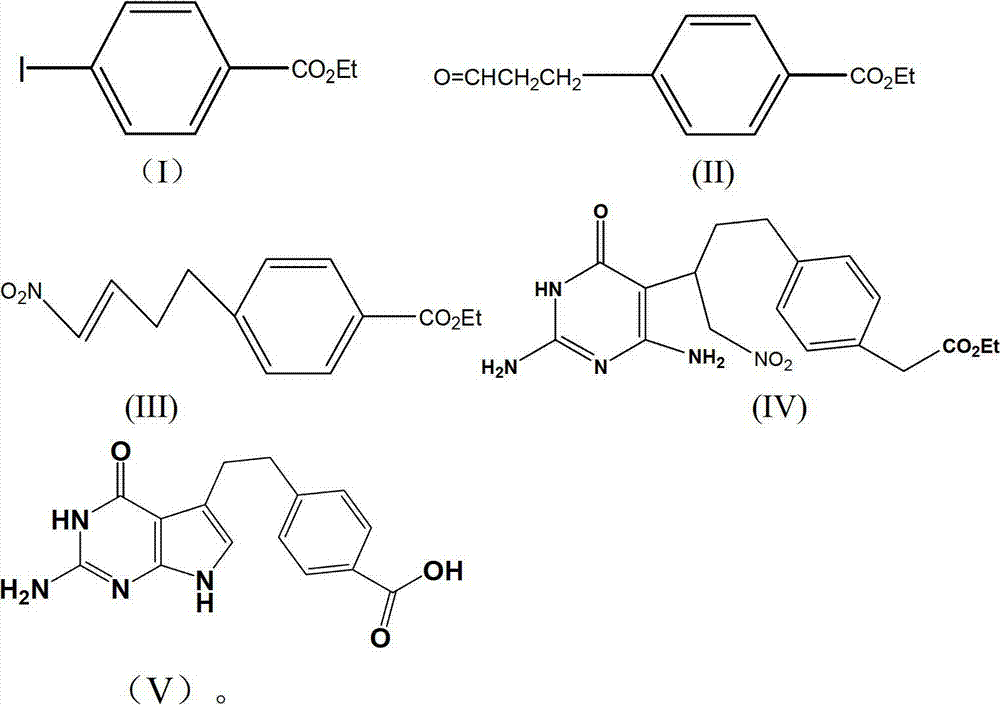
[0022] The preparation of embodiment one, ethyl 4-iodobenzoate
[0023] Mix 24.7g (0.1mol) of 4-iodobenzoic acid, 29ml (0.5mol) of anhydrous ethanol, 250ml of toluene, and 4.8ml (0.03mol) of concentrated sulfuric acid, then stir, and react with water at 110°C for 24 hours, then cool. Wash with 5% Na2CO3 solution (50ml×3), wash with water (50ml×3), dry, filter, concentrate to obtain a yellow liquid, distill under reduced pressure, collect fractions at 60-62°C / 670Pa, and obtain a colorless liquid which is Ethyl 4-iodobenzoate, weighing 25.1g, yield 91.3%.
Embodiment 2
[0024] The preparation of embodiment two, 4-ethoxyformyl phenylpropanal
[0025] 13.8 g (0.05 mol) of ethyl 4-iodobenzoate, Pd (CH 3 COO) 2 0.2g (0.01mol), K 2 CO 3 13.6g (0.1mol), 16.3g (0.05mol) of tetrabutylammonium bromide, mix and stir, add nitrogen protection, add 4.35g (0.075mol) of propenyl alcohol, 150ml of N,N-dimethylformamide, 40℃ After reacting for about 30 hours, concentrate under reduced pressure, pour the residual liquid into 100ml of water, extract the aqueous layer with 50ml×3 petroleum ether, combine the organic layers, dry, filter, concentrate, distill the residual liquid under reduced pressure, and collect 142-144℃ / 670Pa Fractions were obtained as a colorless transparent liquid, namely 4-ethoxyformylbenzaldehyde, with a weighing quality of 9.0 g and a yield of 90.5%.
Embodiment 3
[0026] Example 3, Preparation of 1-nitro-4-(4-ethoxyformylphenyl)-2-butene
[0027] 5.2g (0.025mol) of 4-carboethoxybenzaldehyde prepared in the previous step, 2.3g (0.025mol) of nitromethane, Na 2 CO 3 (0.125mol), stirred at room temperature for 12h, added CH 2 Cl 2 25ml, washed with 1mol / l HCl, then washed with water until neutral, dried, filtered, and concentrated to obtain 6.5g of light yellow solid.
[0028] Take 5.3g (0.02mol) of the reaction product from the previous step, 6.4g (0.063mol) of acetic anhydride, and 8g of pyridine, react at room temperature for 2 hours, pour the reaction solution into 20ml of 2mol / l hydrochloric acid, extract with ether (10ml×3), and combine The organic layer was washed with water until neutral, dried, filtered, concentrated, dissolved the product in 40ml of toluene, added 5.3g of potassium carbonate, stirred and refluxed for 5h, cooled and filtered, the filtrate was washed with water (30ml×3), dried and concentrated to obtain 4.3 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com