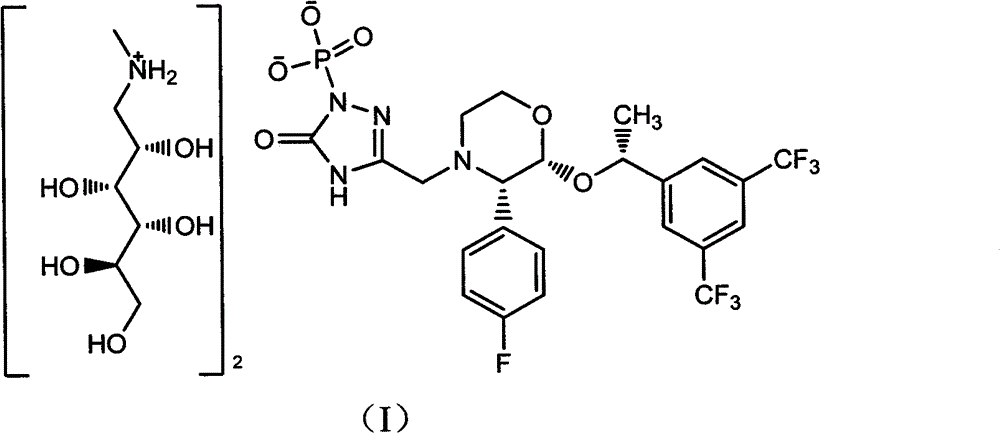
A method for preparing fosaprepitant dimeglumine
The technology of fosaprepitant dimeglumine and step 2 is applied in the field of preparing fosaprepitant dimeglumine, and can solve the problem of being unsuitable for pilot scale scale-up and mass production, having too many monobenzyl esters and low product yield. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
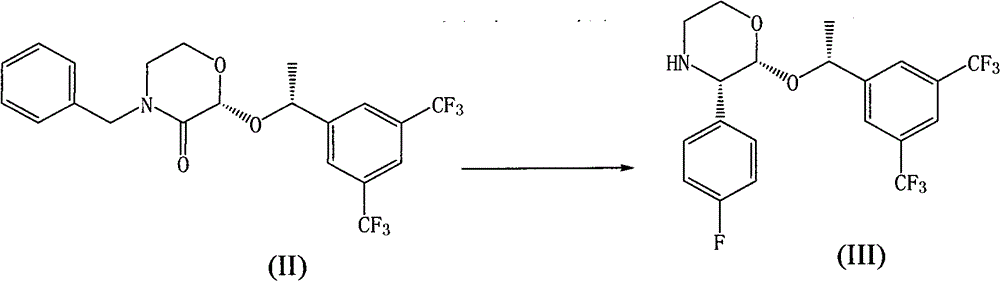
[0037] Example 2 Preparation of compound of formula III
[0038] Add the compound of formula II (13.4g, 30mmol) and tetrahydrofuran (15ml) into a 250ml four-neck flask, stir and cool to 15°C, add 4-fluorophenyl magnesium bromide (1.0M THF solution, 40ml, 40mmol) dropwise, dropwise Stir at room temperature for 40min, add this solution dropwise to ice-cooled methanol (30ml), stir for 15min, add p-toluenesulfonic acid (10.4g, 54.7mmol) in methanol (20ml) solution, 10% Pd / C (0.4g ) And ammonium formate (3.8 g, 60 mmol) until the reaction is complete. Filter, wash with methanol, and concentrate to dryness. Add methyl isobutyl ketone (90 ml), stir, and add a solution of sodium carbonate (9.0 g) / sodium citrate (10.8 g) in water (120 ml). Separate the liquids, extract the aqueous layer with methyl isobutyl ketone (40 ml), combine the organic layers, and wash with water (50 ml). Add concentrated hydrochloric acid (5ml), filter, and evaporate the filtrate to dryness under normal pressur...
Embodiment 3
[0039] Example 3 Synthesis of raw material A
[0040]
[0041] Dissolve sodium (0.23g, 10mmol) in methanol (30ml), add it dropwise to chloroacetonitrile (26.3g, 0.348mol) in methanol (150ml) cooled to 0°C, stir at room temperature for 30min, and add methyl carbazate Ester (30.8g, 0.342mol) and acetic acid (0.6g) were stirred at room temperature for 40min. Concentrate to dry light yellow solid, add ether (150ml), stir under reflux for 30min, stir and cool, filter, wash with ether, and dry to obtain light yellow solid raw material A (56.3g, 99.4%).
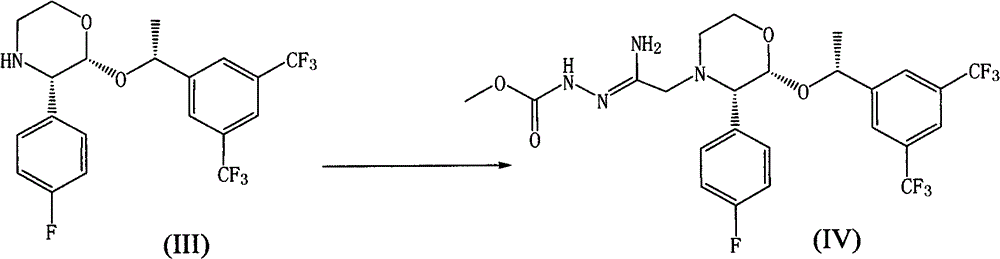
Embodiment 4
[0042] Example 4 Preparation of compound of formula IV
[0043] Add the compound of formula III (10.0g), sodium carbonate (10.2g), dimethyl sulfoxide (20ml), toluene (20ml) into a 250ml reaction flask, stir for 5min in an ice water bath, add raw material A (3.9g) and stir until TLC display After the reaction was complete, toluene (50ml) and water (50ml) were added to separate the layers. The aqueous layer was extracted with toluene (40ml), the organic layers were combined and washed with hot water (50ml) at 50°C. Dry, filter, concentrate to dryness, and directly use in the next reaction.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com