Bimetal nanometer catalyst as well as preparation and application method thereof
A bimetallic nano-catalyst technology, applied in the direction of chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, carbon monoxide or formate reaction preparation, etc., can solve the problems of high loading capacity and high catalyst cost, Achieve the effect of small size, simple and easy to operate in the preparation process, and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
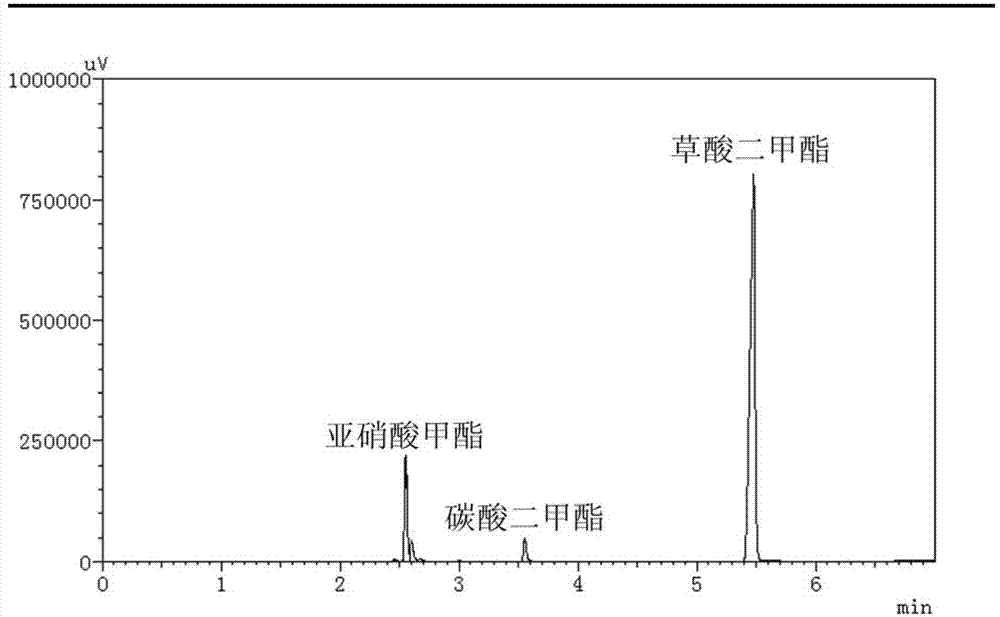
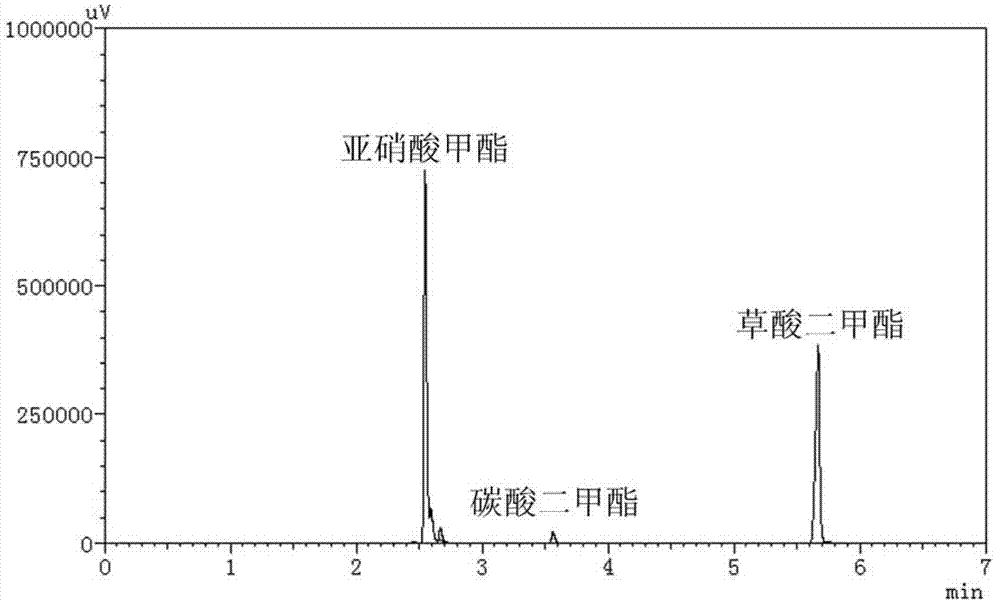
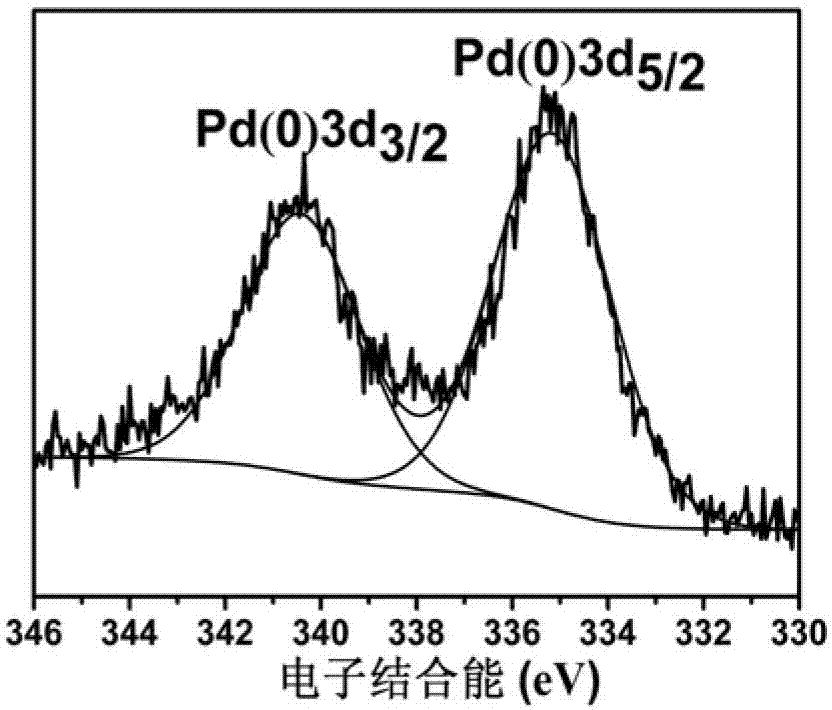
[0041] Weigh 1g of α-alumina and add it to 15ml aqueous solution containing 0.0163g potassium chloropalladate, 0.0170g copper chloride, 0.2220g polyvinylpyrrolidone (PVP), 0.2100g citric acid, stir at room temperature for 0.5 hours, then add A 5ml aqueous solution of 0.0700g ascorbic acid was stirred and reduced at room temperature for 16 hours, and the product was separated by filtration to obtain a filtrate and a solid, and then the solid was washed 6 times with water, absolute ethanol, and acetone, and dried in vacuum at 60°C for 8 hours. The dried solid was activated at 400°C for 3 hours in an atmosphere of pure hydrogen (flow rate 40ml / min), and then cooled to room temperature in an atmosphere of pure hydrogen to prepare the catalyst. Catalyst XPS spectrum see image 3 with Figure 4 , from the electronic binding energy of Pd3d and Cu2P, it can be seen that the valence states of Pd and Cu elements in the catalyst are both zero valence. Catalyst electron microscope photo...
Embodiment 2
[0055] Take by weighing 1g α-alumina and join in the filtrate among the embodiment 1, stir at room temperature 0.5 hour, then add the 5ml aqueous solution that contains 0.0700g ascorbic acid, stir and reduce at room temperature for 24 hours, product filtration separation obtains filtrate and solid, then The solid was washed 6 times with water, absolute ethanol and acetone, and dried under vacuum at 60°C for 8 hours. The dried solid was activated at 400°C for 3 hours in an atmosphere of pure hydrogen (flow rate 40ml / min), and then cooled to room temperature in an atmosphere of pure hydrogen to prepare the catalyst. Catalyst transmission electron microscope photo see Figure 8 , the electron micrograph shows that the Pd-Cu nanoparticles on the catalyst are highly dispersed on the surface of the carrier, the size of the nanoparticles is distributed in the range of 1-5nm, and the average size is 2.7nm. The actual loading of Pd in the catalyst was 0.121% and the actual loading o...
Embodiment 3
[0057] Weigh 1g of α-alumina and add it to 15ml aqueous solution containing 0.0163g potassium chloropalladate, 0.0200g copper acetate, 0.2220g polyvinylpyrrolidone (PVP), 0.2100g citric acid, stir at room temperature for 0.5 hours, then add 0.0700 5ml of aqueous solution of ascorbic acid was stirred and reduced at room temperature for 16 hours, and the product was filtered and separated to obtain a filtrate and a solid, and then the solid was washed 6 times with water, absolute ethanol, and acetone, and dried at 60° C. in vacuum for 8 hours. The dried solid was activated at 400°C for 3 hours in an atmosphere of pure hydrogen (flow rate 40ml / min), and then cooled to room temperature in an atmosphere of pure hydrogen to prepare the catalyst. Catalyst transmission electron microscope photo see Figure 9 , the electron micrograph shows that the Pd-Cu nanoparticles on the catalyst are highly dispersed on the surface of the carrier, the size of the nanoparticles is distributed in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com