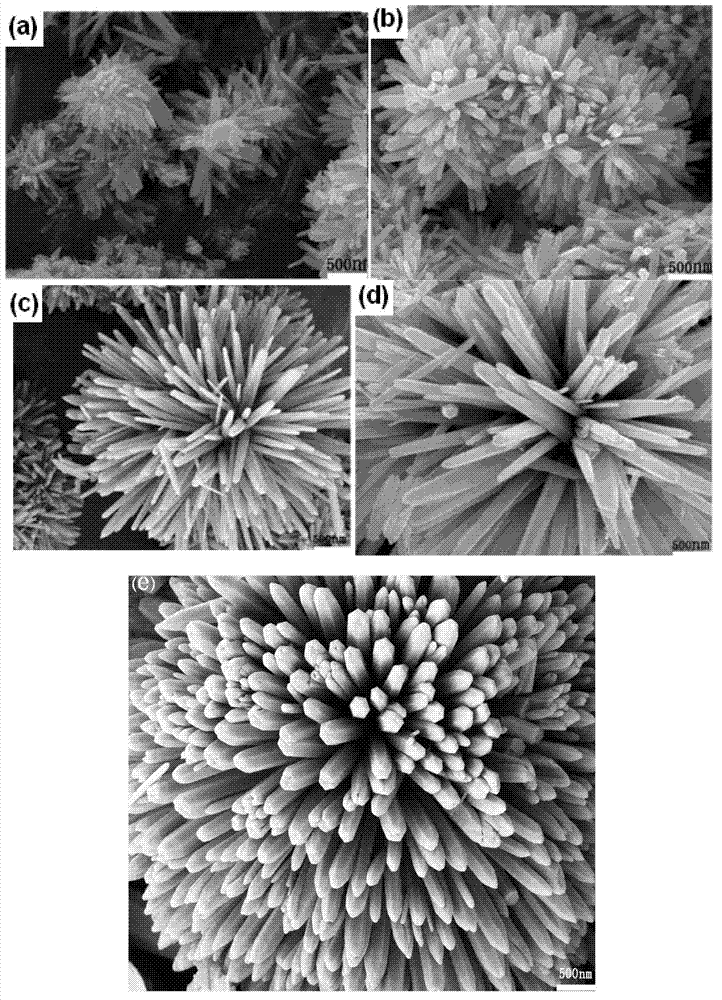
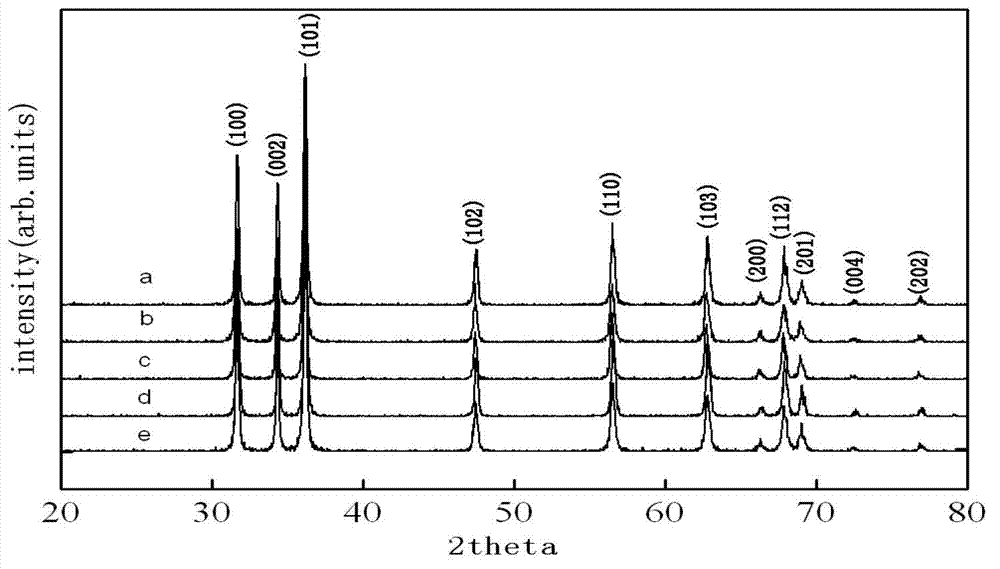
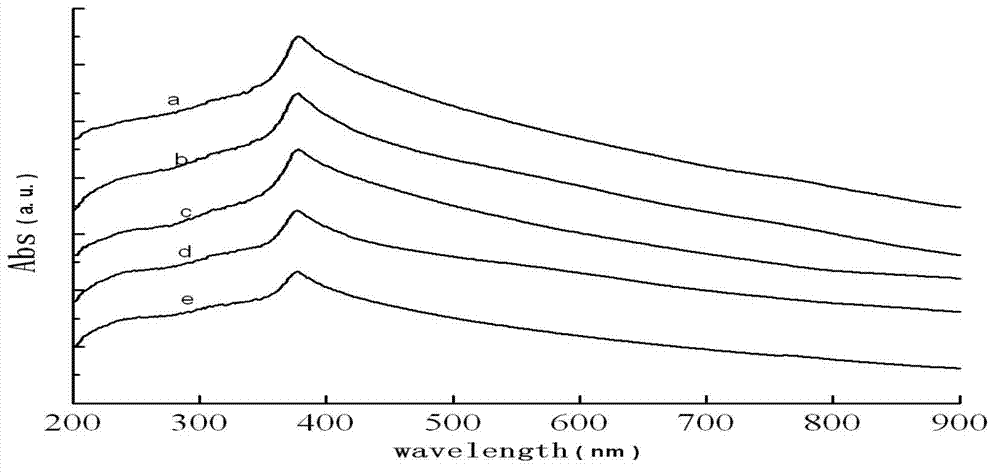
Preparation method of zinc oxide nanorod with strong blue-violet light after being excited
A zinc oxide nanorod, violet light technology, applied in chemical instruments and methods, zinc oxide/zinc hydroxide, nanotechnology, etc., can solve the problem of zinc oxide nanorods with long reaction time, spectral half-height width, poor monochromaticity, etc. problem, to achieve the effect of excellent photocatalytic properties, good monochromaticity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] A method for preparing zinc oxide nanorods excited by strong blue-violet light, comprising the steps of:
[0047] 1) Weigh the zinc source precursor, alkali, weak reducing agent and optional doping element precursor, dissolve in deionized water to obtain solution A;
[0048] 2) Dissolving non-aqueous solvent alcohol and / or ketone and dispersant polyethylene glycol in deionized water to obtain solution B;
[0049] 3) Mix solutions A and B evenly, add a water-soluble surfactant, stir and dissolve, and obtain solution C;
[0050] 4) Transfer the solution C into the reaction kettle, keep it warm at 100-180°C for 0.5-5h, and react to obtain a precipitate;
[0051] 5) The precipitate was separated and dried to obtain zinc oxide nanorods.
[0052] In the above preparation method, preferably, the weak reducing agent is at least one of water-soluble organic amines and fatty acid alcohol amine salts. Wherein the water-soluble organic amine is preferably at least one of alkylamin...
Embodiment 1
[0066] (1) Weigh 0.004mol of Zn(NO 3 ) 2 ·6H 2 O, dissolve in 8ml deionized water, then add 0.03mol NaOH and 0.006mol triethanolamine in sequence, stir to dissolve, add water to 10mL, and obtain solution A;
[0067] (2) Measure 50ml of absolute ethanol, dissolve it in 10ml of deionized water, then add 4.0g of PEG2000, stir to dissolve completely, and obtain solution B;
[0068] (3) Stir and mix solutions A and B, then add 10ml of 0.1mol / L CTAB (trimethylhexadecyl ammonium bromide) solution, stir and mix to obtain solution C;
[0069] (4) Pour solution C into a polytetrafluoroethylene-lined reaction kettle, and react at 100°C for 1 hour with hydrothermal constant temperature;
[0070] (5) After naturally cooling to room temperature, the reactant was taken out and centrifuged, and the precipitate was washed and dried to obtain white powdery zinc oxide nanorods.
Embodiment 2~5
[0072] Examples 2-5 are similar to Example 1, only the reaction temperature is changed, which are 120°C, 140°C, 160°C and 180°C respectively, and other conditions remain unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com