Synthetic method for bactericide of silthiopham
A technology of silthiofen and a synthesis method, applied in the field of organic synthesis, can solve the problems of difficult popularization and application, the total yield is less than 10%, the supply of raw materials and the harsh reaction conditions, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
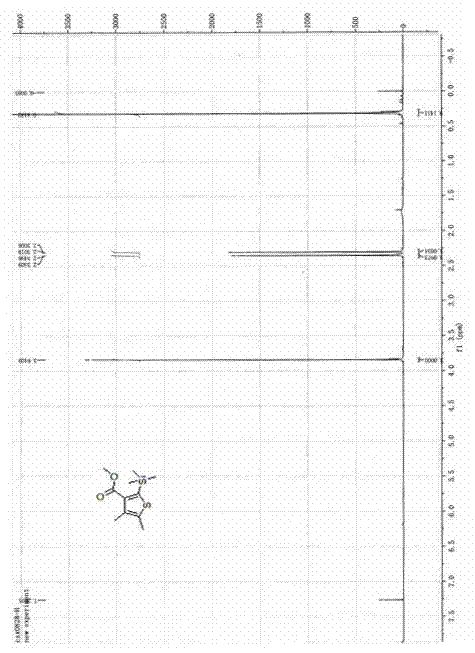
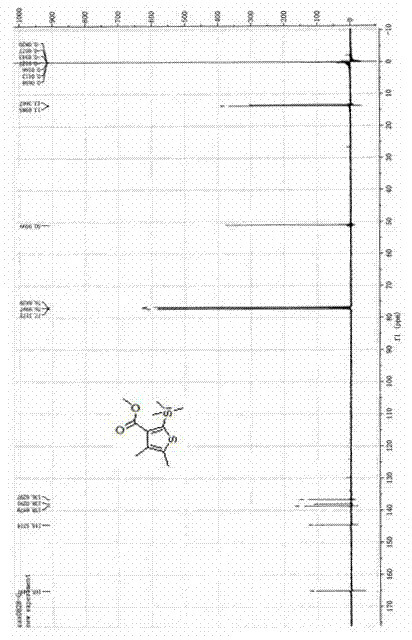
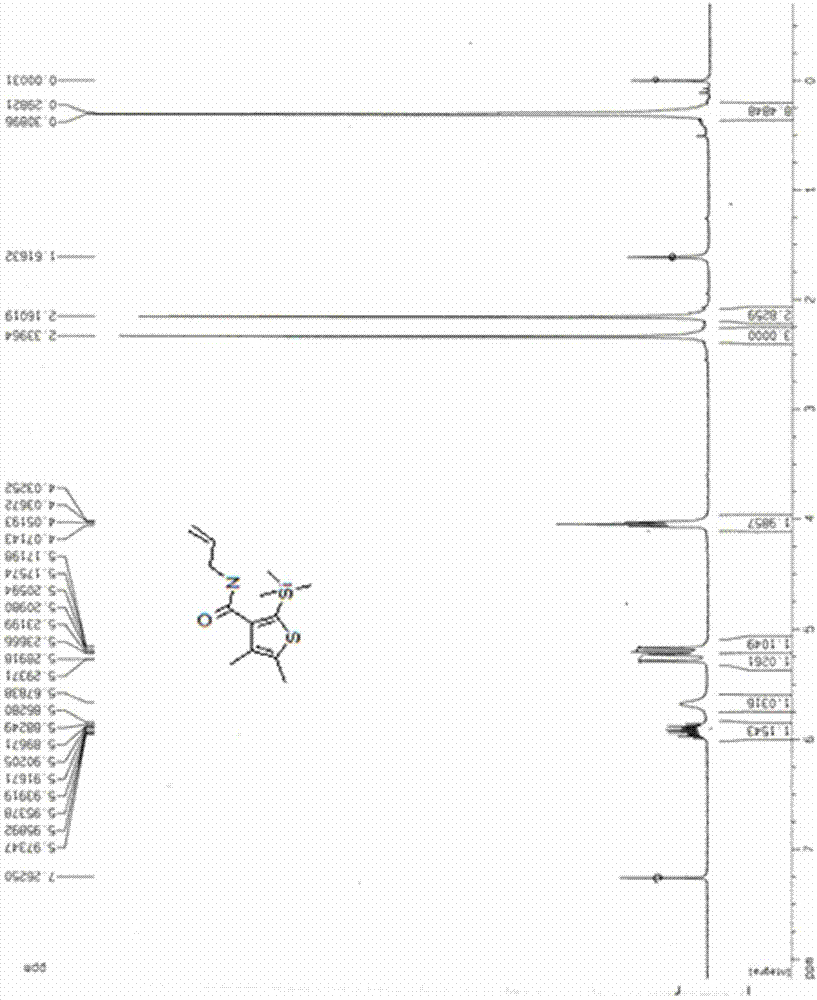
[0020] A kind of synthetic method of bactericide silthiocarb, the method comprises the following steps:
[0021] 1) Preparation of methyl 3-(trimethylsilyl)propiolate
[0022] Put 0.84g (10mmol) of methyl propiolate and 1.41g (13mmol) of trimethylchlorosilane into a three-necked reaction flask containing 5mL of dichloromethane, and slowly add dropwise 5mL of 1.0g (10mmol) of triethylamine The dichloromethane mixed solution was added, and the reaction system was heated to reflux for 3 hours under the protection of nitrogen atmosphere. After the reaction was completed, it was lowered to room temperature, then 5 mL of water was added to stir and mix evenly, and the layers were allowed to stand. After the water phase was separated, the organic phase was dried with anhydrous sodium sulfate and filtered. The mobile phase with a volume ratio of 1:20-50 to petroleum ether was separated by silica gel column chromatography to obtain 1.4 g of light yellow liquid methyl 3-(trimethylsil...
Embodiment 2
[0028] A kind of synthetic method of bactericide silthiocarb, the method comprises the following steps:
[0029] 1) Preparation of methyl 3-(trimethylsilyl)propiolate
[0030] Put 0.84g (10mmol) of methyl propiolate and 2.16g (20mmol) of trimethylchlorosilane into a three-necked reaction flask containing 10mL of toluene, and slowly add dropwise a mixed solution of 5mL of toluene containing 0.9g (10mmol) of morpholine After the addition, the reaction system was heated to reflux for 8 hours under the protection of nitrogen atmosphere. After the reaction was completed, it was lowered to room temperature, then 10 mL of water was added to stir and mix well, and the layers were allowed to stand. After the water phase was separated, the organic phase was dried with anhydrous sodium sulfate and filtered. The mobile phase with a volume ratio of 1:20-50 to petroleum ether was separated by silica gel column chromatography to obtain 1.2 g of light yellow liquid methyl 3-(trimethylsilyl...
Embodiment 3
[0036] A kind of synthetic method of bactericide silthiocarb, the method comprises the following steps:
[0037] 1) Preparation of methyl 3-(trimethylsilyl)propiolate
[0038] Put 0.84g (10mmol) of methyl propiolate and 1.08g (10mmol) of trimethylchlorosilane into a three-necked reaction flask filled with 5mL of N,N-dimethylformamide, slowly dropwise add 2.0g (20mmol) ) triethylamine 5mL N,N-dimethylformamide mixed solution, the addition was completed, and the reaction system was heated to reflux for 12 hours under the protection of nitrogen atmosphere. After the reaction, the solvent N,N-dimethylformamide was distilled off under negative pressure, then cooled to room temperature, washed with 10mL of water, extracted with 30mL of dichloromethane, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was Concentrate, and the concentrated residue is separated by silica gel column chromatography with a mobile phase of ethyl acetate and petroleum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com