Preparation method of tetramethyl biphenyl
A technology of tetramethylbiphenyl and halogenated o-xylene, which is applied in the field of preparation of tetramethylbiphenyl, can solve the problems of difficult reaction control, increased operating environment requirements, violent reaction and the like, and achieves mild and controllable reaction conditions , Simplified production process, mild and controllable conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention provides a preparation method of tetramethylbiphenyl, comprising: A) mixing halogenated o-xylene, metal magnesium, iodine, ether and a transition metal catalyst to react to obtain tetramethylbiphenyl; The molar ratio of ether to magnesium metal is (0.5~3):1.
[0037] Wherein, the halogenated o-xylene is the halogenated o-xylene well known to those skilled in the art, it can be 3-halogenated o-xylene or 4-halogenated o-xylene, or a mixture of both, There are no particular restrictions. The halogen substituents in the halogenated o-xylene in the present invention can be chlorine or bromine, and there is no special limitation.
[0038] The metal magnesium can be metal magnesium well-known to those skilled in the art, and can be magnesium powder, magnesium shavings or magnesium strips, and there is no special limitation. The amount of the magnesium metal is any amount well known to those skilled in the art to maintain the reaction system in a liquid state af...
Embodiment 1
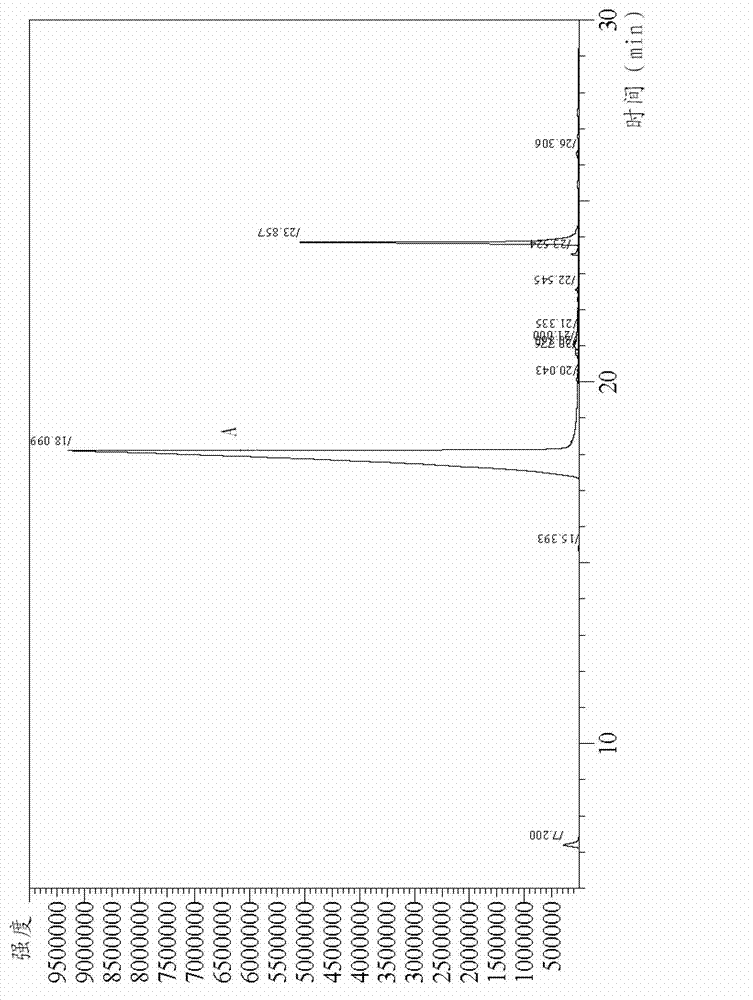
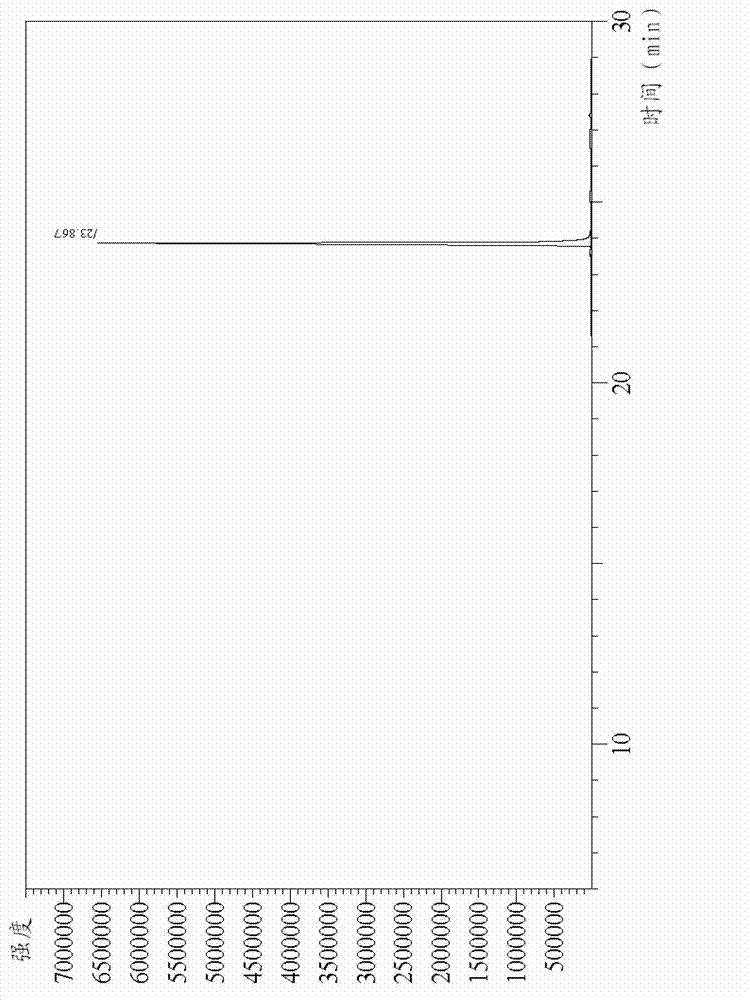
[0062] Replace the air in the 25ml reaction flask equipped with a reflux condenser with nitrogen, and add 0.37g (0.0154mol) magnesium powder, 10ml 3-chloro-o-xylene, 0.01g (3.6 ×10 -6 mol) of iodine and 1.41ml (0.0174mol) of anhydrous THF, stirred and heated to 110°C, the magnesium powder disappeared after 10 hours of reaction, the reaction mixture was cooled to 60°C, and 0.123g (7.7×10 -4 mol) anhydrous ferric chloride, continue to stir and react for 12h, lower the reaction system to room temperature, add 20ml of saturated ammonium chloride solution, stir for 30min, separate the water layer, and analyze the organic layer by gas chromatography to obtain 2,2′,3, 3'-Tetramethylbiphenyl, the yield is 50%, and its gas chromatogram is as follows figure 1 Shown, wherein, A is 3-chloro o-xylene, figure 2 It is the gas chromatogram of the standard sample of 2,2′,3,3′-tetramethylbiphenyl.
Embodiment 2
[0064] Replace the air in a 25ml reaction flask equipped with a reflux condenser with nitrogen, and add 0.37g (0.0154mol) of magnesium powder, 10ml of 4-chloro-o-xylene, 0.01g (3.6 ×10 -6 mol) of iodine and 1.41ml (0.0174mol) of anhydrous THF, stirred and heated to 110°C, the magnesium powder disappeared after 10 hours of reaction, the reaction mixture was cooled to 60°C, and 0.123g (7.7×10 -4 mol) anhydrous ferric chloride, continue to stir and react for 12h, lower the reaction system to room temperature, add 20ml of saturated ammonium chloride solution, stir for 30min, separate the water layer, and analyze the organic layer by gas chromatography to obtain 3,3′,4, 4'-Tetramethylbiphenyl, the yield is 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com