Preparation method of nanometer drug delivery system carrying tanshinone IIA and application thereof
A drug delivery system and a technology containing salvia miltiorrhiza, applied in medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as short half-life, limited use of tanshinone ⅡA, insoluble in water, etc. , to achieve the effect of reducing toxicity, enhancing targeting and therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: The molar ratio of lactic acid and glycolic acid is 50:50, the molar ratio of glycolic acid and lysine is 70:10, the molar ratio of mPEG-PLGA-PLL and cRGD is 1:20, TSIIA and mPEG-PLGA-PLL- The molar ratio of cRGD is 1:20.
Embodiment 2
[0050] Example 2: The molar ratio of lactic acid and glycolic acid is 70:30, the molar ratio of glycolic acid and lysine is 6:10, the molar ratio of mPEG-PLGA-PLL and cRGD is 1:30, TSIIA and mPEG-PLGA-PLL- The molar ratio of cRGD is 1:30.
Embodiment 3
[0051] Example 3: The molar ratio of lactic acid and glycolic acid is 80:20, the molar ratio of glycolic acid and lysine is 50:20, the molar ratio of mPEG-PLGA-PLL and cRGD is 1:50, TSIIA and mPEG-PLGA-PLL- The molar ratio of cRGD is 1:5.
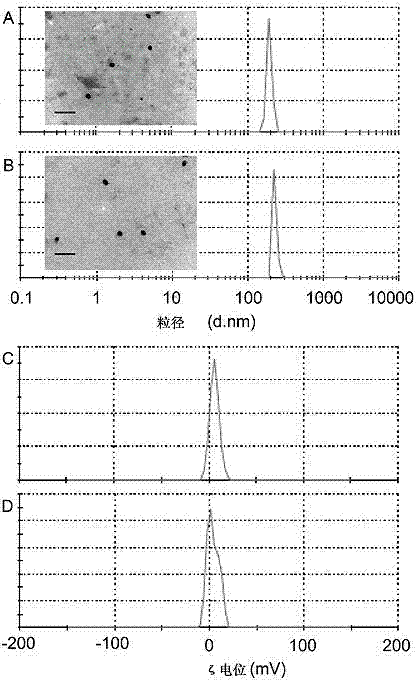
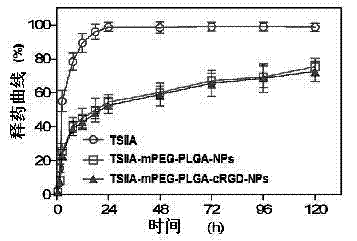
[0052] Take any one of the proportions in Example 1 to Example 3, and prepare polyethylene glycol modified with valine-arginine-glycine-aspartic acid-glutamic acid cyclic peptide (cRGD) according to the following method Tanshinone IIA (TSIIA)-loaded nanoparticles supported by monomethyl ether-polylactic-glycolic acid-polylysine (mPEG-PLGA-PLL) polymer.
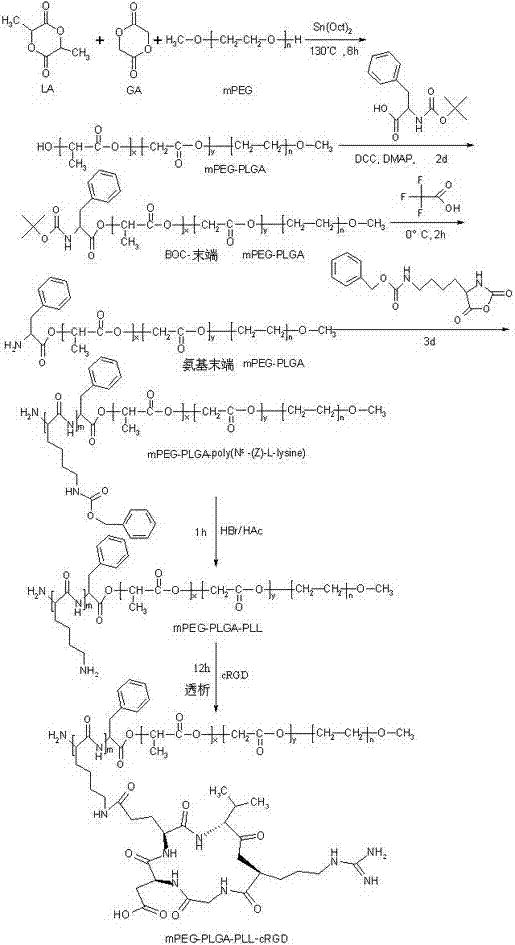
[0053] Step 1, such as figure 1 cRGD-modified mPEG-PLGA-PLL-cRGD was prepared as indicated. Among them, h is hour, d is day, LA is lactide, Sn(Oct) 2 is stannous octoate, mPEG is polyethylene glycol monomethyl ether, PLGA is polylactic acid / glycolic acid, DCC is dicyclohexylcarbodiimide, DMAP is 4-dimethylaminopyridine, BOC is phenylalanine, HAc is acetic acid, HBr is hydrobromic acid, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com