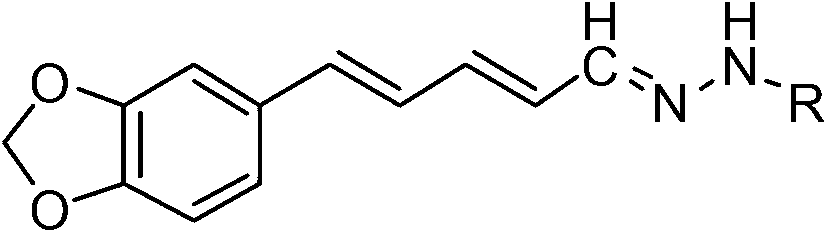
Piperine hydrazone or acylhydrazone or sulfonyl hydrazone derivative substances and application for preparing a botanical insecticide
A technology of piperine hydrazone and sulfonyl hydrazone, which is applied in the direction of pesticides, plant growth regulators, applications, etc., and can solve the problems that the activity of piperine and its derivatives has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
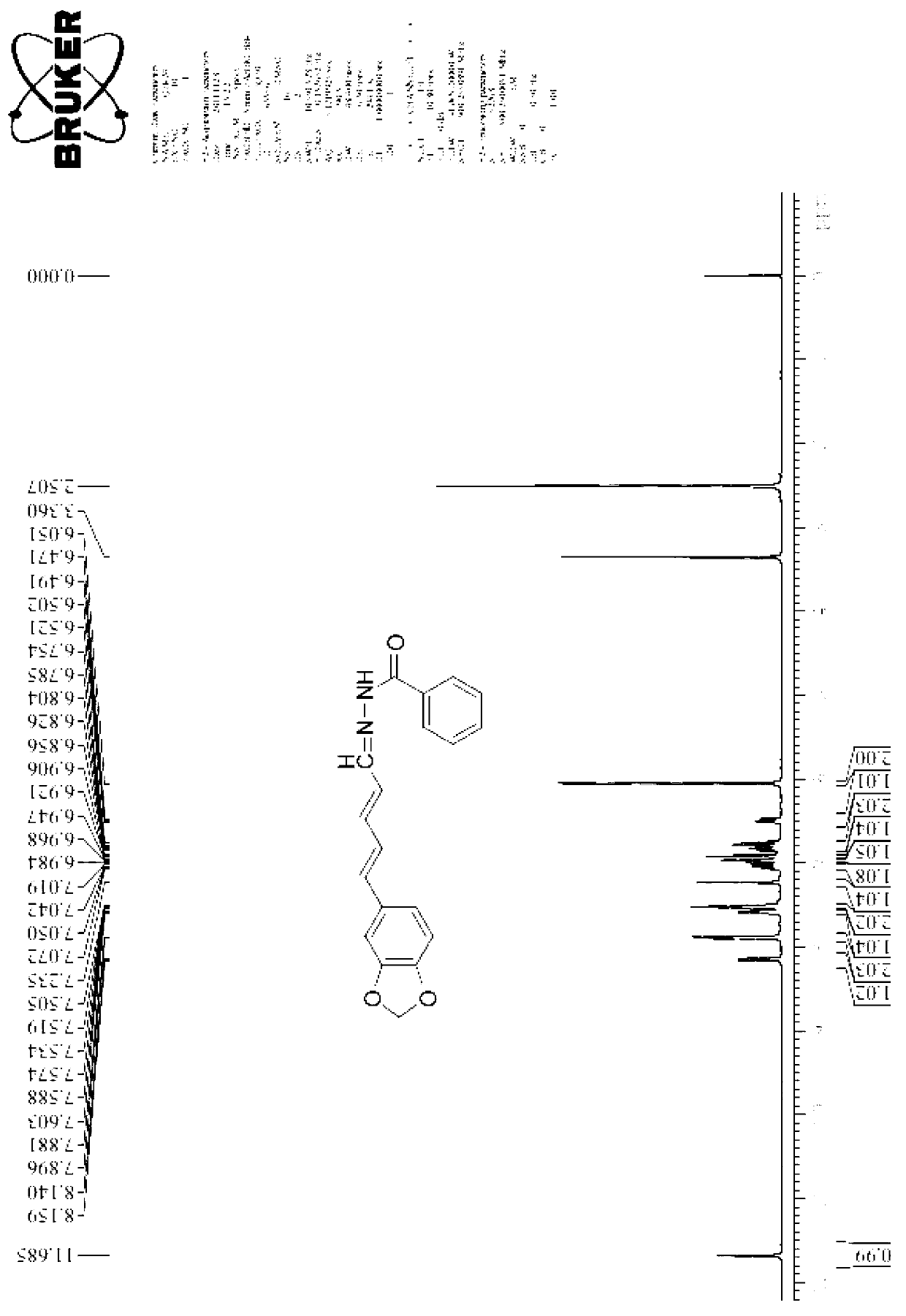
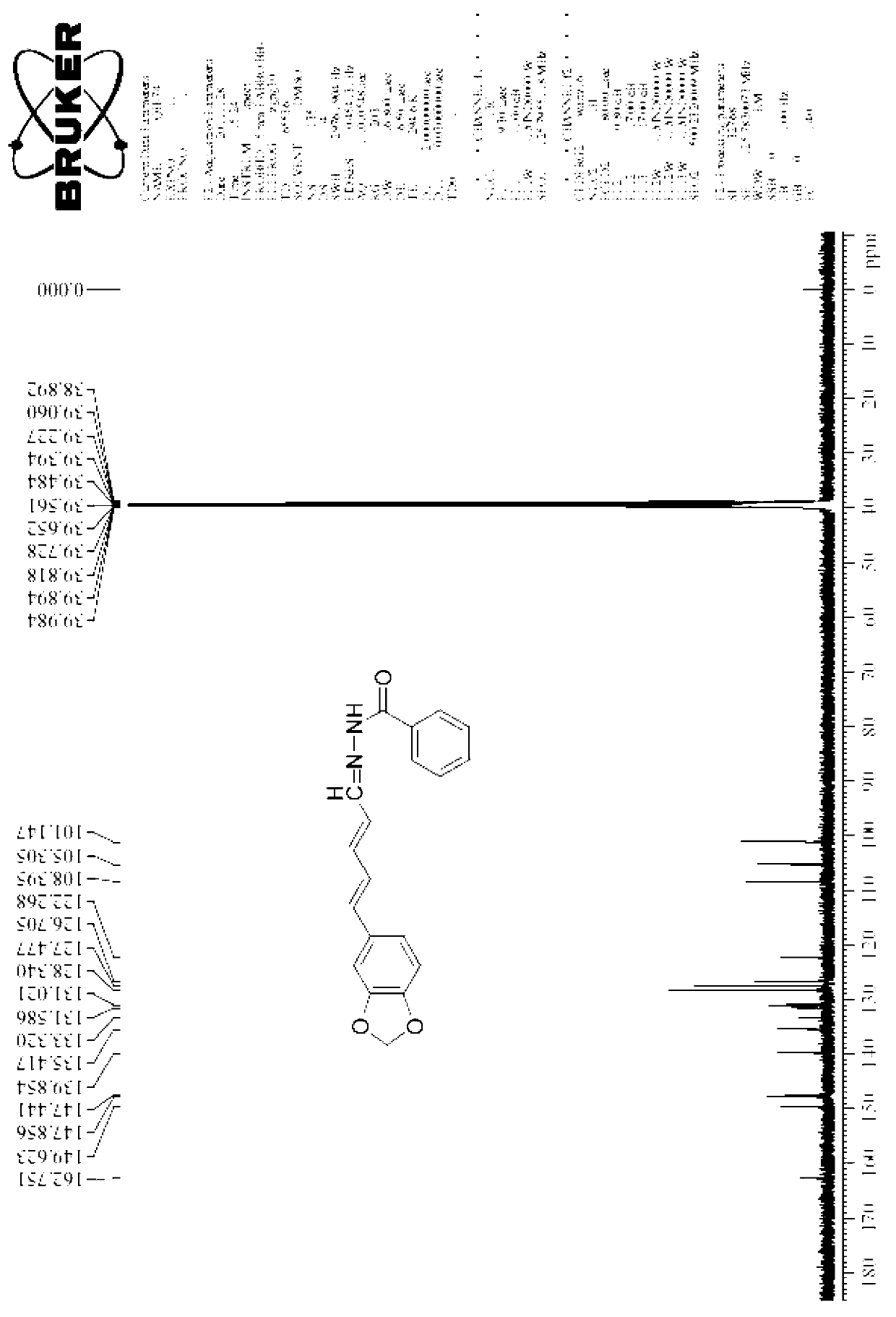
[0032] 1. Products: piperine acid, piperine ester, piperine alcohol, piperine aldehyde and compound 1-33 (see the following content for details on the physical and chemical properties of each compound)
[0033] 2. Preparation method:
[0034] The following is the synthetic route of piperine acid:
[0035] Dissolve a certain amount of potassium hydroxide in 95% ethanol, then add piperine to the above reaction solution, reflux in an oil bath for a certain period of time, cool and filter at room temperature to obtain a solid, dissolve it in a certain amount of water, and adjust the pH with hydrochloric acid The value is acidic, suction filtered, the filter cake is washed with a small amount of water and dried to obtain the desired pure product.
[0036]
[0037] Physical and chemical properties of piperine acid:
[0038] 1), yellow solid, melting point 218°C.
[0039] 2) Mass spectrometry (ESI-MS) characteristics of piperine acid:
[0040] Using electrospray ionization: m / ...
Embodiment 2
[0231] Embodiment 2: bioassay experiment
[0232] 1. Insects to be tested: armyworm larvae in the early 3rd instar, provided by the insect breeding room of the Pollution-free Pesticide Research Center of Northwest A&F University.
[0233] 2. Samples and reagents:
[0234] The samples are: toosendanin, piperine acid, piperine ester, piperine alcohol, piperine aldehyde and compound 1-33 prepared in the examples. The solvent was acetone, analytically pure, from Chengdu Kelong Chemical Reagent Factory.
[0235] 3. Bioassay method:
[0236] Using the method of adding small leaf butterfly: spread a layer of filter paper on the bottom of a petri dish with a diameter of 9 cm, and add water to moisturize it. Pick 10 3rd instar prophase armyworm larvae with the same size and robustness from each dish. Weigh 5 mg of toosendanin, piperine acid, piperine ester, piperine alcohol, piperine aldehyde and compound 1-33 prepared in the examples and add 5 ml of acetone to prepare a drug solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com