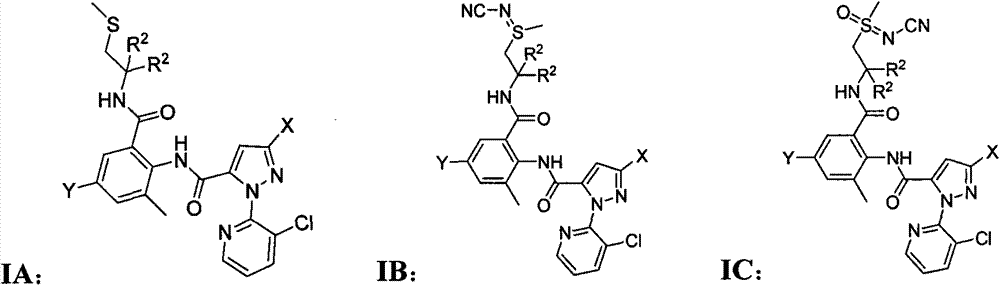
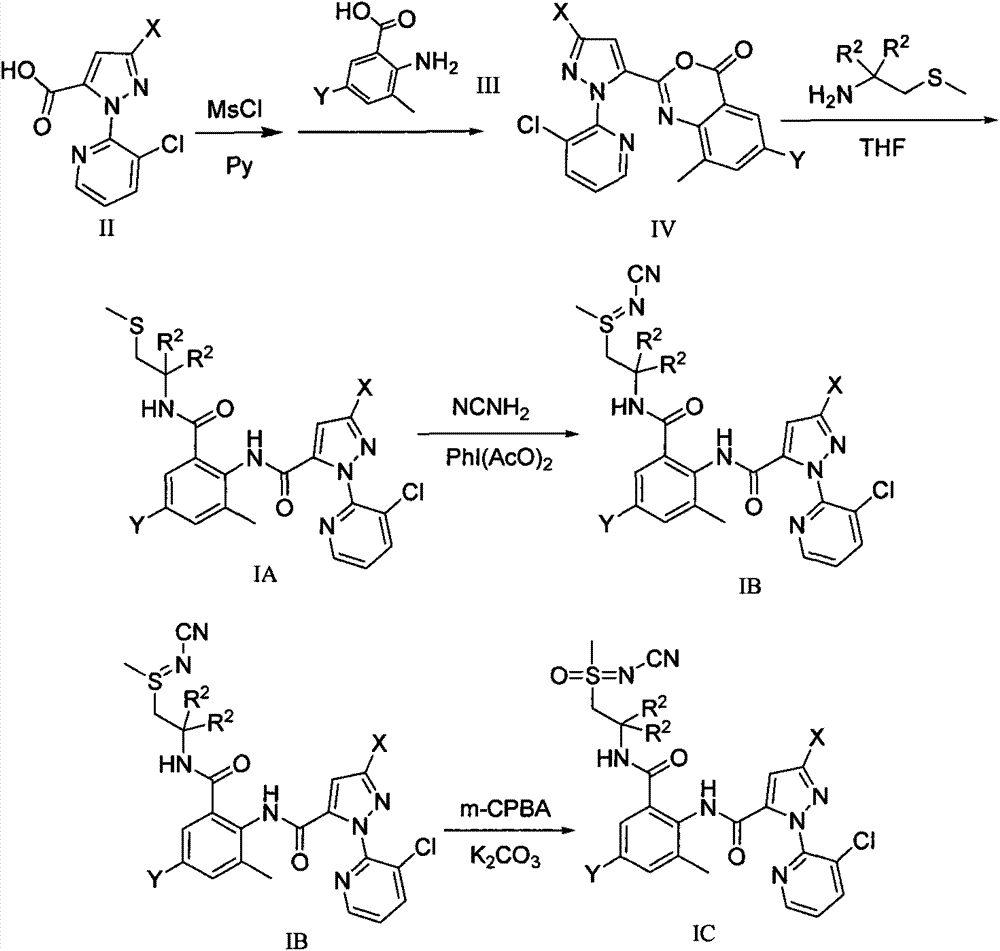
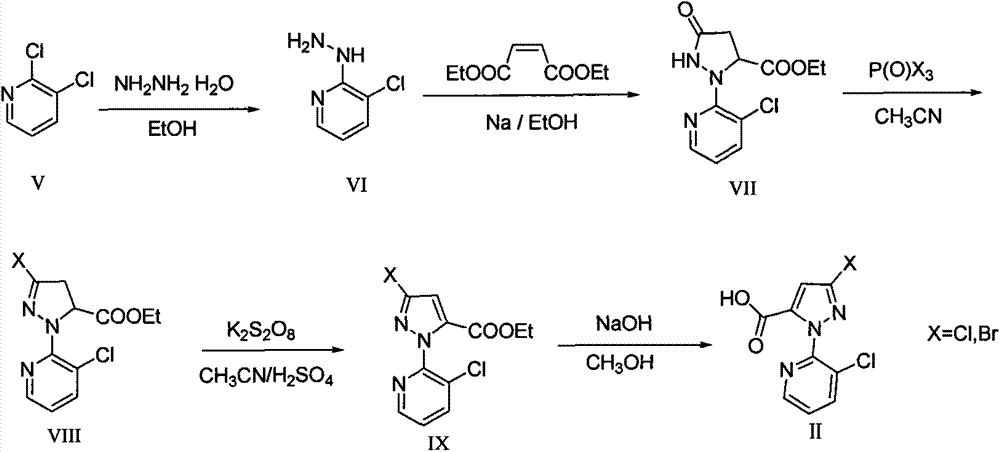
O-formylamino benzamide derivative containing N-cyano sulfone(sulfur) imine and preparation method and use thereof
A technology of o-formamidobenzamide and cyanosulfone, applied in the field of o-formamidobenzamide derivatives, can solve the problem of no activity exceeding the activity of the target molecule parent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of 3-methyl-5-halo-2-aminobenzoic acid III:
[0055]
[0056] In a 100 ml round bottom flask, add 3.0 g, 20 mmol 3-methyl-2-aminobenzoic acid X, 30 ml DMF, 6.7 g, 30 mmol NCS (or 30 mmol NBS), and stir at reflux for 3 hours , pour the reaction solution into ice water, acidify with dilute hydrochloric acid to pH=6, filter, and wash the obtained solid with a small amount of ethanol to obtain 2-amino-3-methyl-5-halo-benzoic acid III, gray solid, more than 4.6 g , the yield is greater than 83%. Y is selected from: chlorine, bromine, and the 2-amino-3-methyl-5-halo-benzoic acid III is selected from the group consisting of -2-amino-3-methyl-5-chlorobenzoic acid, 2-amino-3 -Methyl-5-bromo-benzoic acid; where, 2-amino-3-methyl-5-chlorobenzoic acid 1 H NMR (400MHz, DMSO-d 6 ): δ: 7.96(s, 1H), 7.56(d, 1H), 7.23(d, 1H), 2.11(s, 3H); 2-amino-3-methyl-5-bromobenzoic acid 1 H NMR (300MHz, DMSO-d 6 ): δ: 7.70 (s, 1H), 7.31 (s, 1H), 2.10 (s, 3H).
Embodiment 2
[0058] 3-Halo-1-(3-chloropyridin-2-yl)-N-(4-halo-2-methyl-6-((2-(methylthio)alkyl)carbamoyl)benzene base)-1H-pyrazole-5-carboxamide preparation
[0059] (1) Synthesis of 3-chloro-2-hydrazinopyridine VI:
[0060]
[0061] Add 50 mmoles of 2,3-pyridine V, 100 mmoles of hydrazine hydrate, and 50 ml of ethanol into a 100 ml three-necked round-bottomed flask, heat and reflux at 80 degrees Celsius for 20 hours, evaporate the solvent under reduced pressure, and weigh the obtained solid with ethanol Crystallization gave 6.5 g of needle-like crystals of 3-chloro-2-hydrazinopyridine VI, with a yield of 90%.
[0062] (2) Synthesis of ethyl 5-oxo-2-(3-chloropyridin-2-yl)pyrazoline-3-carboxylate VII:
[0063]
[0064] Add 100 ml of absolute ethanol to a 250 ml single-necked round-bottom flask, add 1.77 g or 55 mmol of sodium metal in batches, the temperature rises slightly, and form a homogeneous transparent solution after stirring for 10 minutes; add 7.1 g or 50 mmol of 3-chloro ...
Embodiment 3
[0081] 1-(3-Chloropyridin-2-yl)-3-halo-1H-pyrazole-5-formyl-2-methyl-4-halo-6-(2-((N-cyanomethyl Synthesis of (sulfimino)alkyl)carbamoyl)aniline IB
[0082]
[0083]In a 50 ml three-neck round bottom flask, add 0.53 g, namely 1.0 mmol 3-halo-1-(3-chloropyridin-2-yl)-N-(4-halo-2-methyl-6-(2 -(methylthioalkyl)carbamoyl)phenyl)-1H-pyrazole-5-carboxamide IA was added to 30 ml of dry tetrahydrofuran, stirred, and cooled to 0°C in an ice-water bath. Then add 0.16 g, 4 mmol cyanamide and 0.33 g, 1.0 mmol iodobenzene acetate respectively, stir at this temperature for 3 hours, then stir overnight at room temperature, the next day, the reaction solution is directly decompressed to remove the solvent, and the residue is subjected to 200~ Purified by 300-mesh silica gel column chromatography to obtain white solid IB, the eluent was ethyl acetate, and the physical and chemical parameters of IB are shown in Table 1. The 1-(3-chloropyridin-2-yl)-3-halo-1H-pyrazole-5-formyl-2-methyl-4-ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com