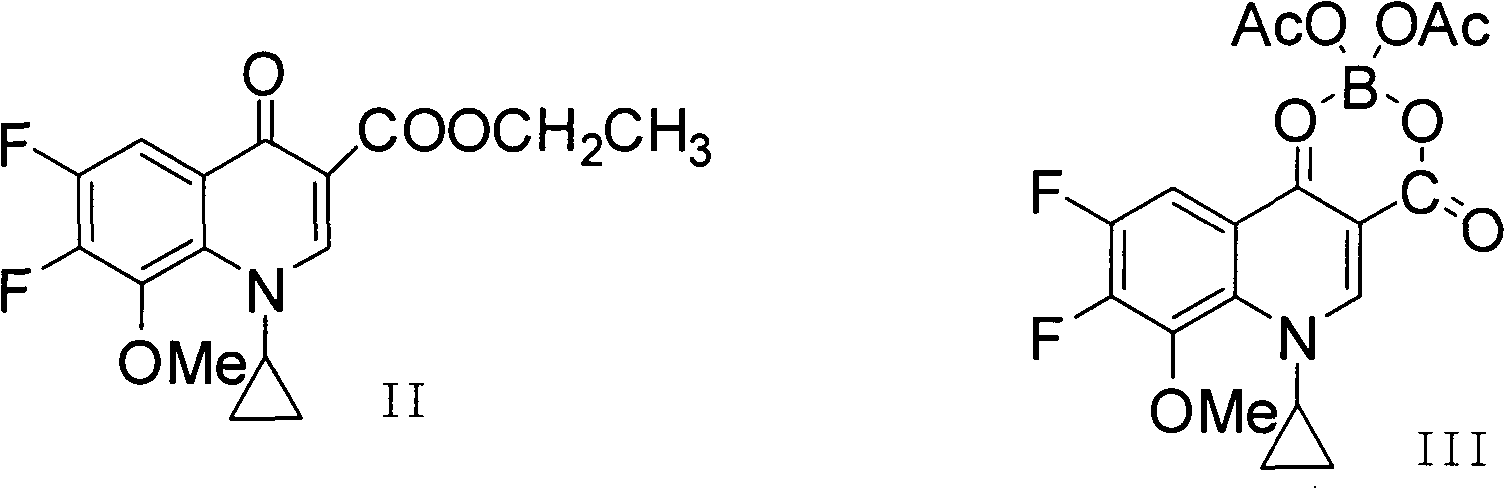Synthesis method of high-purity moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and its synthesis method, which is applied in the field of synthesis of quinolone broad-spectrum antibacterial drugs, can solve the problems of unreported synthesis method of anhydrous moxifloxacin hydrochloride, low purity and large loss of moxifloxacin hydrochloride, and achieve Human drug safety, mild conditions, good reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
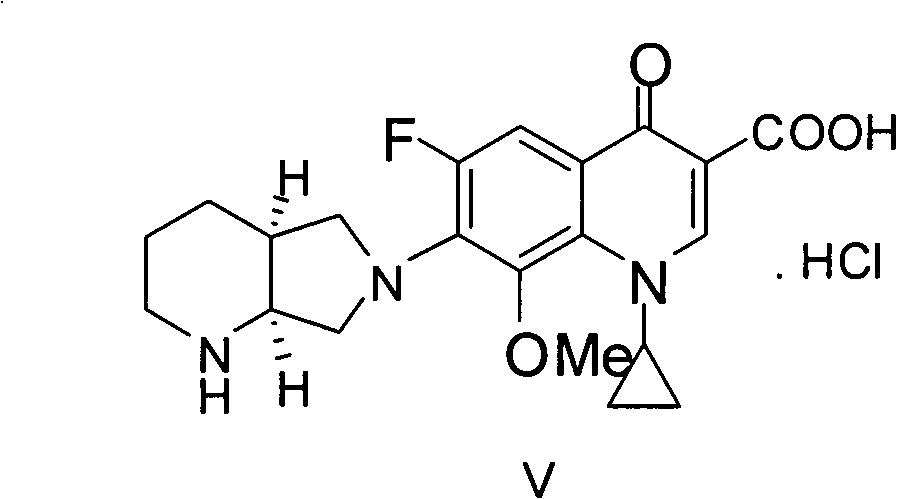
[0039] Dissolve 50 g of crude moxifloxacin hydrochloride in aqueous sodium hydroxide solution with a concentration of 5 mol / L. At this time, the pH is 14, then extract three times with 60 ml of ethyl acetate, remove the organic phase, and add concentrated hydrochloric acid to the water phase to adjust the pH to 1. , then crystallize and filter to obtain a filter cake, and then use 300ml of methanol aqueous solution with a volume percentage of 90% to recrystallize the filter cake. Specifically, add the obtained filter cake and methanol aqueous solution to the reaction flask, and reflux for 15 to 30 minutes under stirring conditions. Filtrate while hot, then distill to recover the solvent, crystallize at 0-5°C, and finally filter and dry under reduced pressure at 40°C for 36 hours to obtain 48g of moxifloxacin hydrochloride with a yield of 96%.
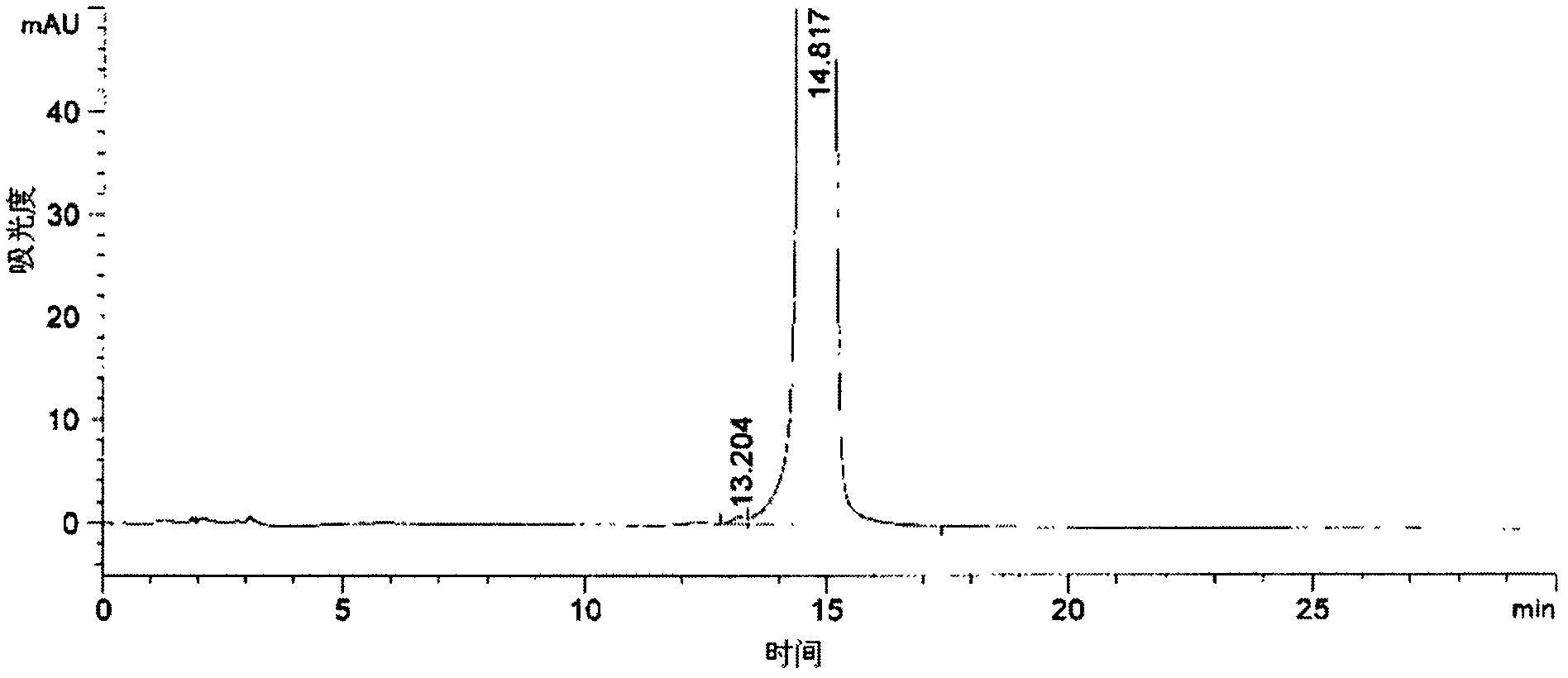
[0040]Adopt Agilent 1100 type high performance liquid chromatography (HPLC) to analyze the purity of moxifloxacin hydrochloride product...
Embodiment 2
[0043] Dissolve 50g of moxifloxacin hydrochloride crude product with an aqueous potassium hydroxide solution with a concentration of 2mol / L. At this time, the pH is 13, then extract three times with 60ml of dichloromethane, remove the organic phase, and add concentrated hydrochloric acid to the water phase to adjust the pH to 2 , then crystallize and filter to obtain a filter cake, and then use 250ml of 85% methanol aqueous solution to recrystallize the filter cake, specifically, add the obtained filter cake and ethanol aqueous solution to the reaction flask, and reflux for 30 to 40 minutes under stirring conditions, Filtrate while hot, then distill to recover the solvent, crystallize at 5-10°C, finally filter, and dry under reduced pressure at 60°C for 28 hours to obtain 44g of moxifloxacin hydrochloride with a yield of 88%, of which, the water content is 0.8%, and the HPLC purity is is 99.97%.
Embodiment 3
[0045] Dissolve 50g of crude moxifloxacin hydrochloride in ammonia water with a concentration of 3mol / L. At this time, the pH is 11, then extract it three times with 60ml of methyl tert-butyl ether, remove the organic phase, and add concentrated hydrochloric acid to the water phase to adjust the pH to 3. , filter, then crystallize and filter to obtain a filter cake, and then use 500ml of 95% ethanol aqueous solution to recrystallize the filter cake, specifically, add the obtained filter cake and methanol aqueous solution to the reaction flask, and reflux for 40 to 60 minutes under stirring , filtered while it was hot, then distilled to recover the solvent, crystallized at 0-5°C, finally filtered, and dried under reduced pressure at 90°C for 16 hours to obtain 47g of moxifloxacin hydrochloride with a yield of 94%, of which the water content was 0.8%, HPLC The purity is 99.96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com