Protopanoxadiol biosynthesizing method and bacterial strain for producing protopanoxadiol
A protopanaxadiol and synthase technology, applied in the field of genetic engineering, can solve the problems of complex extraction technology, long growth cycle, and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Construction of dammarenediol synthase expression vector
[0042] The dammarenediol synthase gene GenBank: AB265170.1 in the ginseng genome was codon optimized according to the codon preference of Pichia pastoris GS115, and the optimized dammarenediol synthase gene sequence is SEQ ID NO: 1, SEQ ID NO:1 was synthesized by Shanghai Qinglan Biotechnology Co., Ltd.
[0043] Design restriction restriction sites AsuII and XbaI at both ends of the gene fragment. Using primer F respectively:
[0044] 5'-TACTTCGAAATGGCCTGGAAGCAAAAAAGGTGCTC-3' and R:
[0045] 5'-GCCTCTAGATTAAATTTTCAACTGCTGATGTTAG-3' was amplified by PCR. PCR amplification conditions: 94°C, 5min; 94°C, 30S; 58°C, 30S, 30 cycles; 72°C, 2.5min; 72°C, 10min.
[0046]The PCR amplified product and Pichia pastoris expression plasmid pPicZa were digested with AsuII and XbaI, and digested overnight at 37°C, and the digested product was recovered and purified on agarose gel. Design a ligation system with a...
Embodiment 2
[0047] Example 2 Construction of recombinant expression engineering bacteria for dammarenediol synthase
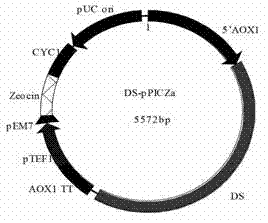
[0048] Positive transformants were cultured in LB medium at 37°C and 200rpm for 8h, the cells were collected, and the plasmid DS-pPICZa was extracted. SacI single-digestion treatment, overnight at 37°C, PCR purification and recovery, collected in 15ul of water, and stored at 4°C until use.
[0049] Prepare Pichia pastoris GS115 competent cells, add SacI-treated DS-pPICZa plasmid, mix well, place in ice bath for 5 minutes, transfer to electroporation cup, 1.98kv, 5.8ms, electric shock transformation. Add 1ml of sorbitol that was pre-cooled in advance, and incubate at 30°C for 30min. Collect the bacteria by centrifugation, add 1ml of YPD medium, rejuvenate at 200rpm at 30°C for 1 hour, collect the bacteria, smear on 100ug / ml bleomycin resistance screening YPD plate, culture at 30°C for 48h-72h. 800ug / ml bleomycin resistance was used to screen high-copy transformants, colon...
Embodiment 3P450
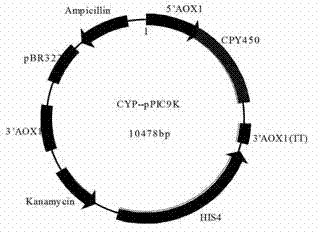
[0053] Example 3 Construction of P450 Cytochrome Synthetase Expression Vector
[0054] The P450 cytochrome synthase (CYP) gene GenBank: JN604537.1 in the ginseng genome was codon optimized according to the codon preference of Pichia pastoris. The optimized gene sequence is SEQ ID NO: 2, which was provided by Shanghai Qing Synthesized by Lan Biotechnology Co., Ltd.
[0055] The upstream and downstream restriction sites of the CYP gene fragment were designed to be BamHI and NotI, respectively. Design PCR primer F:
[0056] 5'-CAT GGATCC ATGGTTTTGTTCTTTTCCCTTTCAC-3' and R:5'-
[0057] GAAT GCGGCCGC TTAGTTATGAGGGTGAAGGTGAATAG-3'PCR amplification conditions are shown in the table below:
[0058]
[0059] The PCR product and its Pichia pastoris expression plasmid Ppic9k were digested with BamHI and NotI, and digested at 37°C for 4 hours. After the fragments were recovered by cutting the agarose gel, they were ligated overnight at 16°C. Prepare Escherichia coli DH5a compe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com