Sulbenicillin sodium injection
A technology for sulfobenicillin sodium and injection, which is applied in the field of high-purity D(-)-sulfobenicillin sodium powder injection and its preparation, can solve the problems of unfavorable labor protection, low product purity, large adverse reactions, etc. Low cost, good curative effect, and the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of sterile sulbenicillin sodium raw materials:
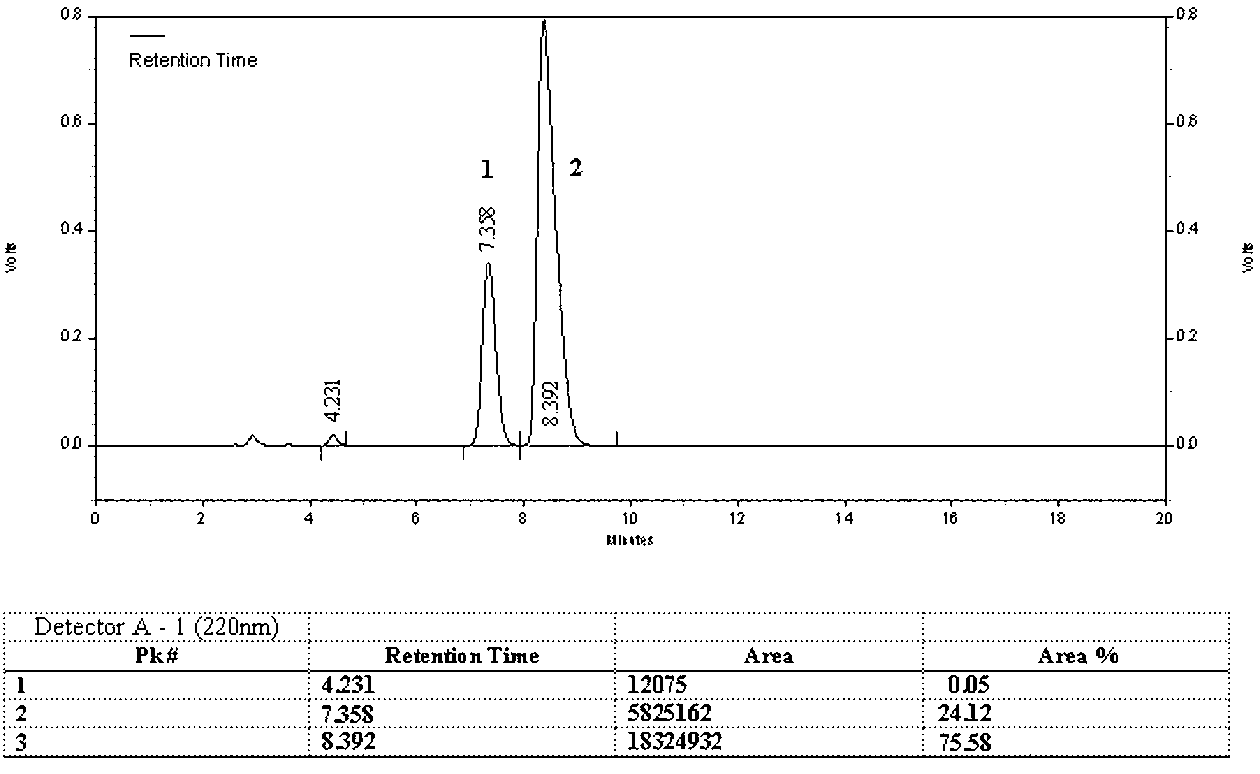
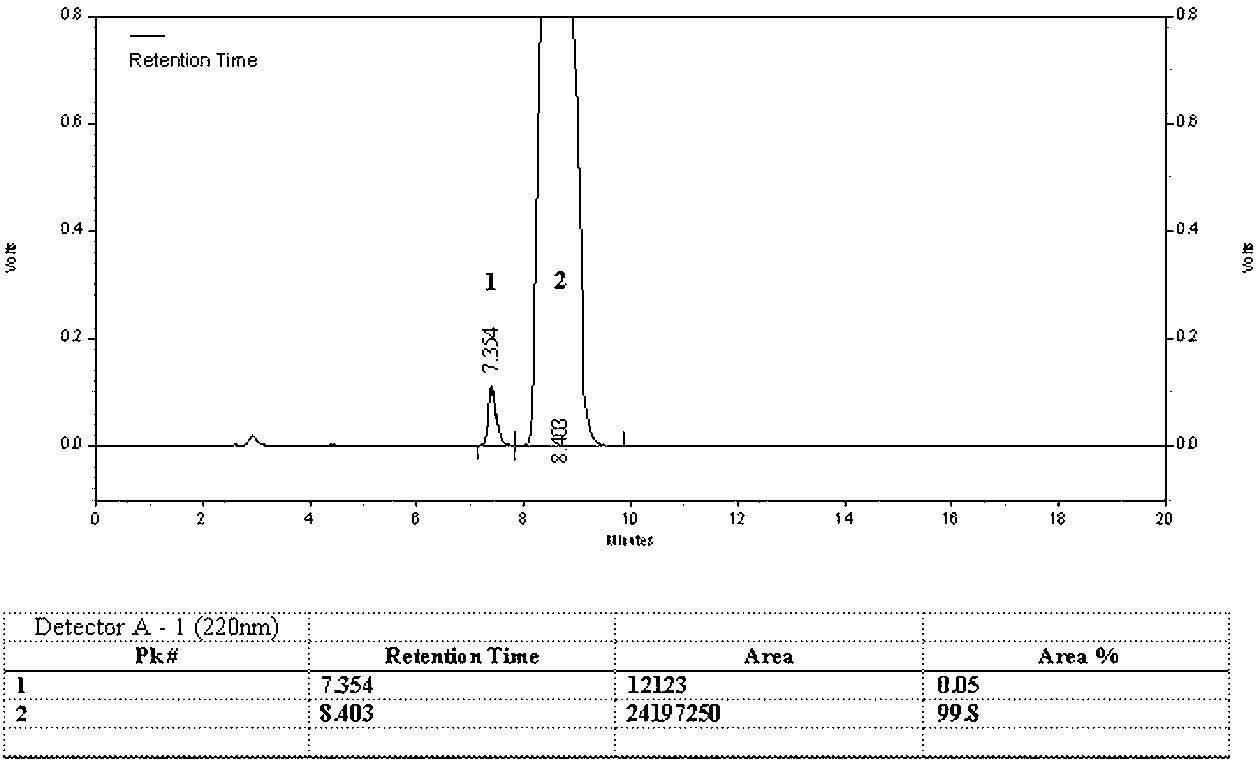
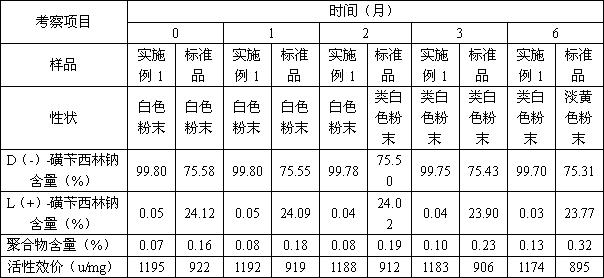
[0065]1) Preparation of D(-)-α-sulfophenylacetic acid: Weigh 50kg of (±)-α-sulfophenylacetic acid, dissolve it in 500L of water, add 40.58kg of D-p-hydroxyphenylglycine (the molar ratio of feed is 1: 1.05), heated to 60°C, stirred for 2 hours, cooled to 20°C, filtered, collected the filtrate, adjusted the pH to 6.5 with 1mol / L sodium hydroxide, filtered, and collected the solid D-p-hydroxyphenylglycine in another device for reuse, the filtrate Concentrate to dryness, add 250L ethanol, adjust pH to 1.5 with concentrated hydrochloric acid, stir for 1 hour, add 50L acetone, precipitate solid matter, filter to obtain 21.55kg of D(-)-α-sulfophenylacetic acid, yield 86.2%, HPLC content 96.8%;
[0066] 2) Preparation of D(-)-α-sulfophenylacetyl chloride: Dissolve 20kg of D(-)-α-sulfophenylacetic acid and 12L of triethylamine in 200L of methyl tert-butyl ether, stir and add chlorination Sulfoxide 6.7L, co...
Embodiment 2
[0069] Example 2 Sulbenicillin Sodium Powder Injection (0.5g specification)
[0070] Sulbenicillin Sodium Sterile Powder 5kg
[0071] -------------------------------------------------- --
[0072] 10,000 made
[0073] Preparation method:
[0074] (1) Bottle washing and sterilization: Take the soda-lime glass injection bottle, rinse it with drinking water, and irradiate it with ultraviolet light for 30 minutes, then enter the bottle washing machine, rinse it with water for injection, blow off the residual water with compressed air, and enter the 330°C tunnel The oven is dried, sterilized, and cooled for later use;
[0075] (2) Cleaning and sterilization of rubber stoppers: take bromobutyl rubber stoppers, rough wash with purified water, rinse, then finely wash with water for injection, dry and sterilize for later use;
[0076] (3) Clean and sterilize the aluminum cap for the injection bottle: take the aluminum cap and put it into the alumi...
Embodiment 3
[0081] Example 3 Sulbenicillin Sodium Powder Injection (1.0g specification)
[0082] Sulbenicillin Sodium Sterile Powder 10kg
[0083] -------------------------------------------------- ----
[0084] 10,000 made
[0085] Preparation method:
[0086] (1) Bottle washing and sterilization: Take the soda-lime glass injection bottle, rinse it with drinking water, and irradiate it with ultraviolet light for 30 minutes, then enter the bottle washing machine, rinse it with water for injection, blow off the residual water with compressed air, and enter the 330°C tunnel The oven is dried, sterilized, and cooled for later use;
[0087] (2) Cleaning and sterilization of rubber stoppers: take bromobutyl rubber stoppers, rough wash with purified water, rinse, then finely wash with water for injection, dry and sterilize for later use;
[0088] (3) Clean and sterilize the aluminum cap for the injection bottle: take the aluminum cap and put it into the alu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com