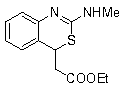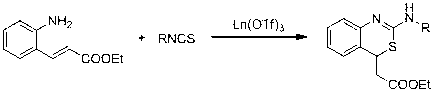Method for preparing 2-amino benzothiazine
A technology of aminobenzene and thiazine, which is applied in the field of catalytic preparation of 2-aminobenzothiazine, can solve the problems of high price of noble metal catalysts, limitation of the application of existing technologies, and complicated raw materials used, so as to facilitate purification and simple post-treatment , easy-to-obtain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: Catalyst Yb (OTf) 3 Synthesis
[0034] In the reaction flask, an excess of ytterbium oxide with a purity of 99.9% was added to an aqueous solution of trifluoromethanesulfonic acid (the volume ratio of acid to water was 1:1), and heated to reflux for 60 minutes; the unreacted ytterbium oxide was removed by filtration, and the Remove water under pressure to obtain ytterbium trifluoromethanesulfonate with crystal water; finally vacuum dehydrate at 180°C-200°C for 36-48 hours to obtain the desired ytterbium triflate Yb(OTf) 3 .
[0035] Other Ln(OTf) 3 Catalyst can refer to the preparation method of Example 1.
Embodiment
[0036] Example : La(OTf) 3 Synthesis of 2-Aminobenzothiazine by Catalytic Reaction of Ethyl o-Aminocinnamate and Phenyl Isothiocyanate
[0037] Weigh La(OTf) into a dry reaction flask 3 (0.0059 g, 0.01 mmol ), added ethyl o-aminocinnamate (0.1912 g, 1 mmol ) and phenylisothiocyanate (0.1622 g, 1.2 mmol ) in sequence, stirred at 50°C for 4 hours after mixing, added water Terminate the reaction, extract three times with ethyl acetate, dry the extract with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and finally perform flash column chromatography on a silica gel column (eluent: ethyl acetate:petroleum ether=1:10) to obtain White solid product, 84% yield.
[0038] The theoretical molecular formula of the obtained product and the main NMR test data are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0039]
[0040] 1H NMR (400 MHz, CDCl 3 ) δ7.57 (d, J = 8.0 ...
Embodiment 3
[0041] Embodiment three: Nd(OTf) 3 Preparation of 2-aminobenzothiazine by catalyzing the reaction between ethyl o-aminocinnamate and phenylisothiocyanate
[0042] Weigh Nd(OTf) into a dry reaction flask 3 (0.0059 g, 0.01 mmol ), added ethyl o-aminocinnamate (0.1912 g, 1 mmol ) and phenylisothiocyanate (0.1622 g, 1.2 mmol ) in sequence, stirred at 50°C for 4 hours after mixing, added water Terminate the reaction, extract three times with ethyl acetate, dry the extract with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and finally perform flash column chromatography on a silica gel column (eluent: ethyl acetate:petroleum ether=1:10) to obtain White solid product, 85% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com