Benzo terthiophene compound as well as preparation method and usage thereof
A compound, trifluoromethyl technology, applied in the field of benzotrithiophene compounds and their preparation, can solve the problems of difficult purification of conjugated polymers, poor device repeatability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] Preparation method of benzotrithiophene compound
[0087] The benzotrithiophene compounds of the present invention can be prepared by conventional synthesis methods known to those skilled in the art. In a class of preferred embodiments of the present invention, the preparation method of the compound comprises the step of performing Stille coupling reaction on the compound of formula IV and the compound of formula V in the presence of a catalyst, thereby forming the compound of formula I.
[0088]
[0089] In the formula, the definition of X is the same as above; R 7 Is methyl, n-butyl or tert-butyl; L is I or Br.
[0090] The catalyst can be a conventional catalyst used in the coupling reaction, preferably a palladium catalyst or a combination of a palladium catalyst and a ligand.
[0091] Palladium catalysts include but are not limited to: PdM 2 , Pd(MeCN) 2 Cl 2 , Pd(PhCN) 2 Cl 2 , Pd(dppf)Cl 2 , Pd(dppe)Cl 2 , Pd(dppb)Cl 2 , Pd(dppp)Cl 2 , Pd(dppm)Cl 2...
Embodiment 5
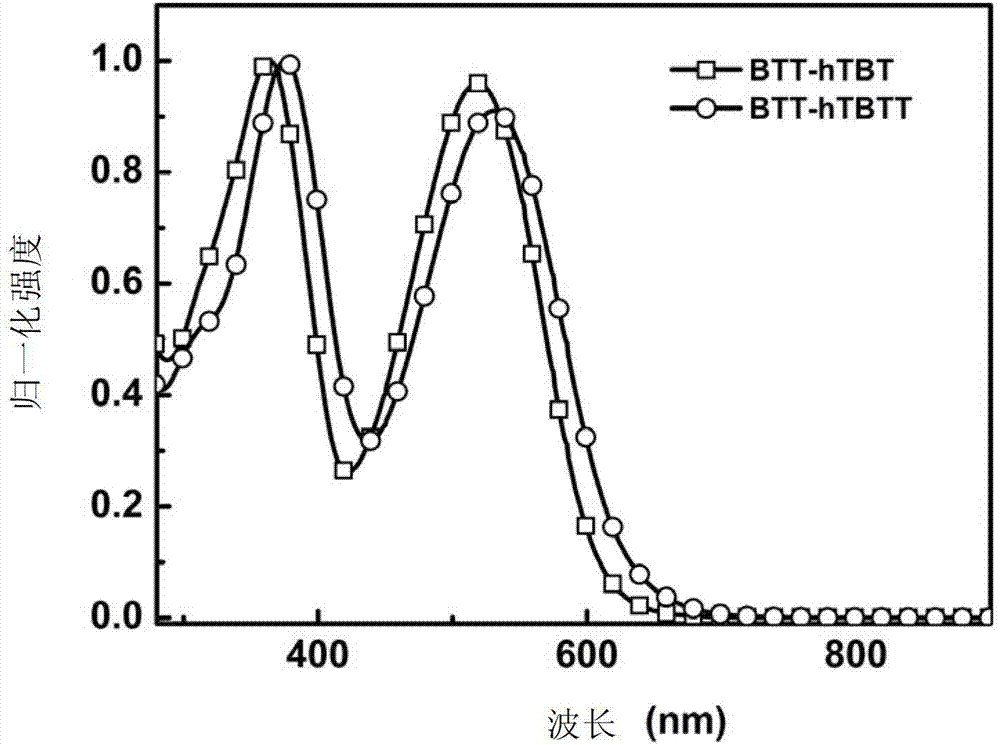
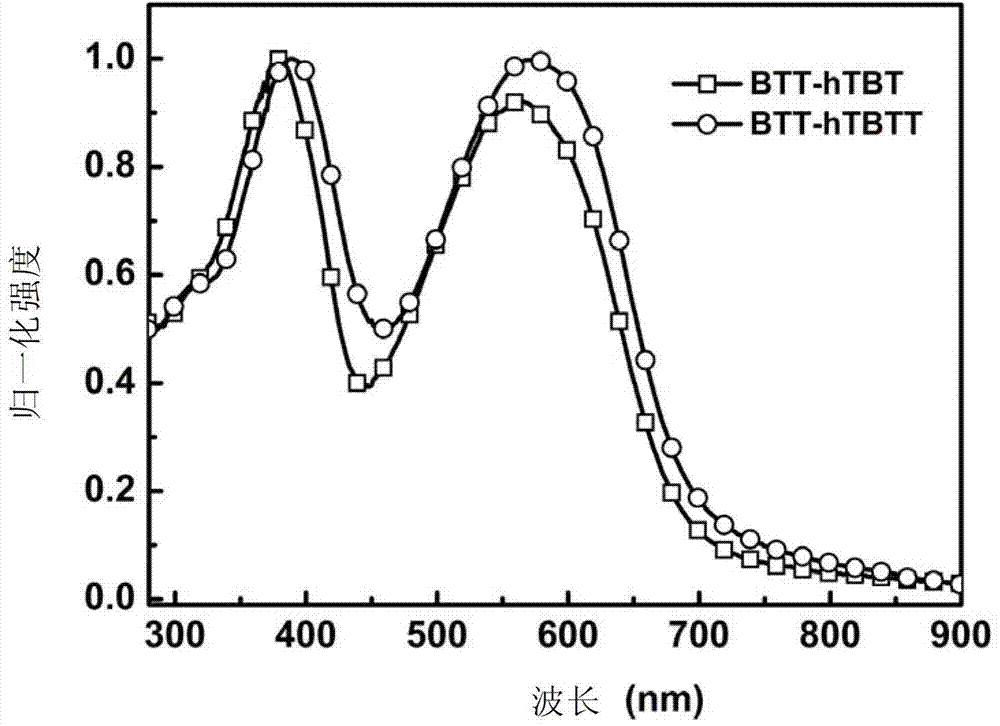
[0251] Embodiment 5: UV-visible absorption spectrum test
[0252] The ultraviolet-visible absorption spectrum test of compound BTT-hTBT and BTT-hTBTT in chlorobenzene solution:
[0253] The ultraviolet-visible absorption spectrum of above-mentioned compound is carried out on the ultraviolet-visible spectrophotometer, wherein the used solvent of solution absorption spectrum is chlorobenzene, and concentration is 10 -5 mol / L; Take 2mL of the solution of each compound and add it to a quartz cuvette, put it into a mapada UV-3300 UV-Visible spectrophotometer, after calibrating the baseline and blank, test the absorption of the solution in the range of 200-1000nm spectrum. The test results show (such as figure 1 shown), the compound solution described in this application has good absorption in the visible light region, which meets the basic light absorption requirements of organic solar cell donor materials. At the same time, the increase of the branched chain conjugated system m...
Embodiment 6
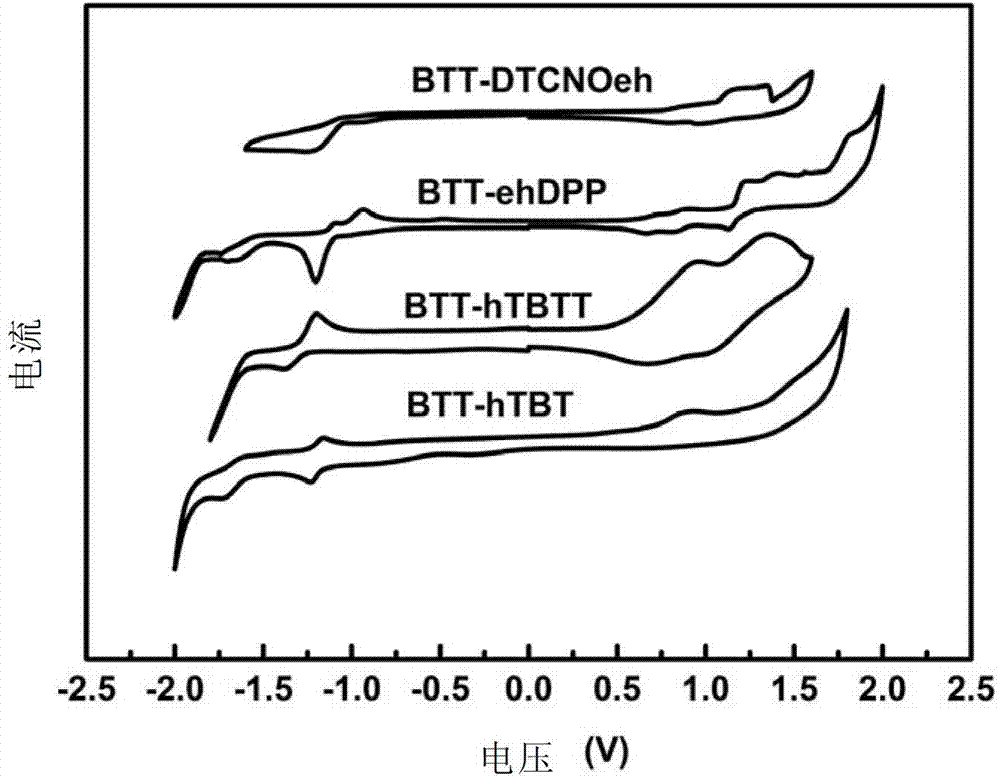
[0256] Embodiment 6: Cyclic voltammetry test
[0257] Cyclic voltammetry tests on compounds BTT-hTBT, BTT-hTBTT, BTT-ehDPP and BTT-DTCNOeh:
[0258] The energy level of the highest occupied orbital (HOMO) and the lowest unoccupied orbital (LUMO) of the molecule can be calculated by measuring the redox potential of the molecule by cyclic voltammetry. In this embodiment, electrochemical workstations are used to test electrochemical properties. The electrolytic cell is a three-electrode system (a glassy carbon electrode is a working electrode, a glass wire electrode is an auxiliary electrode, and a silver / silver chloride electrode is a reference electrode). Ferrocene was used as internal standard, dried dichloromethane or acetonitrile was used as solvent, 0.1mol / L tetrabutylammonium hexafluorophosphate was used as supporting electrolyte, and the scanning speed was 50mV / S. Under the protection of argon, scan to obtain the cyclic voltammetry curve (such as image 3 shown), the HO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com